Translate this page into:
Fetal Blood Transfusion: The Saviour
DR. J.G. JOLLY ORATION delivered during NAMSCON 2017 at Sri Guru Ram Das Institute of Medical Sciences & Research, Sri Amritsar, Punjab.
Correspondence:Brigadier (Dr.) Devendra Arora, Professor & Head, Department of Obstetrics & Gynaecology, Spl in Maternal Fetal Medicine, Command Hospital (SC), Pune-411040, (Maharashtra). Mob : 09540667660. Email: drdarora@hotmail.com
Abstract
The purpose of this oration is to discuss the modality of highly specialized Intra vascular fetal blood transfusion and its various sites to perform fetal blood transfusion with the role of middle cerebral artery-peak systolic velocity (MCA-PSV), as measured by Doppler ultrasound, in managing fetal anemia in Rh-alloimmunized pregnancies. Intra-uterine fetal blood transfusion was performed in such anemic fetuses to tide over the crisis of fetal immaturity till considered fit for extra-uterine survival. Rh-alloimmunized pregnancies with or without hydrops reporting to our tertiary care institute from January, 2005 to December, 2015 were screened by Doppler ultrasound to estimate MCA-PSV to detect fetal anemia. During follow-up, if the fetus developed MCA-PSV values more than 1.5 MoM for the gestational age, fetal blood sampling through cordocentesis was performed to confirm fetal anemia. This was followed by intra-uterine fetal blood transfusion to all the anemic fetuses at the same sitting. The neonatal outcome was evaluated by recording gestational age at the time of delivery, duration of gestational time gained, and need for blood transfusion in the neonatal period. A total of 226 Rh-alloimmunized pregnancies were evaluated. Three hundred ninety six intra-uterine fetal blood transfusions were performed. In their neonatal period, 137 neonates received blood transfusion. Intra-uterine fetal death occurred in 11 fetuses out of which 7 were grossly hydropic fetus. Favorable neonatal outcome was recorded in the rest including 42 hydropic fetuses. The clinical outcome of these pregnancies justifies the use of Doppler studies of MCA-PSV in detecting fetal anemia as these were found to correlate well. Intra-uterine fetal blood transfusion in the anemic fetuses is the only hope of prolonging pregnancy salvaging such fetuses.
Keywords
Intra-uterine fetal blood transfusion
Rh-alloimmunization
hemolytic disease of fetus and newborn
fetal anemia
MCA-PSV
cordocentesis
neonatal outcome.
Introduction
The hemolytic disease of the fetus and newborn (HDFN) has now largely supplanted the more clumsy and less euphonic name “erythroblastosis fetalis”. The essential underlying pathology is an active hemolysis of the Rh D positive fetal red cells before, at, and after birth. The three conditions hydrops fetalis, icterus gravis neonatorum, and hemolytic anemia of the newborn are recognized to differ only in the degree and are related to one disease process. Dr. Ian Donald (Regius Professor of Midwifery at Glasgow University) quotes that the recognition of Rh factor, its clinical importance and practical applications of this knowledge in fetal therapy as one of the great romances of modern research where theory, observation and practice have, in the space of few years, pieced themselves together to form a coherent picture.
HDFN secondary to maternal rhesus (Rh) isoimmunization or alloimmunization was once a major contributor to perinatal morbidity and mortality. The Rh factor, a blood group D antigen is the main cause of hemolytic anemia of the newborn. The outcome is manifested by hemolytic anemia and/or jaundice in the newborn or hydrops (severe edematous swelling) or a stillborn baby. The disease is produced when a mother is of blood group Rh D negative, the father is Rh D positive and the conceived baby inherits the father's Rh D positive blood group. There is feto-maternal hemorrhage during pregnancy. The baby's blood group is incompatible with that of the mother and generates an immunological response against paternally derived red blood cell antigen D foreign to the mother but inherited by the fetus in the form of antibodies from placental cross over. These maternal antibodies response escapes in first pregnancy but in subsequent pregnancies, if the fetus is Rh D positive, the antibodies cross the placenta and destroy the baby's Rh positive red blood cells resulting frequently fetal anemia, hemolytic jaundice of new born, hydrops, stillbirth, etc. This hemolytic disease can be prevented if the Rh D negative mother has not been sensitized (developed antibodies). This can be accomplished by giving an injection of sterilized blood product called Rh immunoglobulin. Today, administration of Rh immunoglobulin has markedly decreased the prevalence of fetal hemolytic disease such that less than three cases occur in every 1000 live births (1). Failure of Rh immuno-prophylaxis still occurs as a result of inadequate use of Rh immune prophylaxis after potential sensitizing events and administration of inadequate dose. The clinician must be alert to detect Rh alloimmunization because it still constitutes significant proportion of perinatal mortality. Rhesus disease will have a favourable outcome in most instances if properly managed. The rarity of this condition warrants consideration of consultation or referral to a maternal-fetal medicine specialist. The introduction of high-resolution real time ultrasound and the development of intra uterine fetal blood transfusion performed intra-vascular has improved the prognosis optimally even in severely alloimmunized women with hydropic fetus. The reduction in perinatal deaths due to HDFN has also occurred as a result of the great advances made in neonatal care and in the management of the affected fetus.
Evaluation for the presence of maternal anti-D antibody should be undertaken at the first prenatal visit by performing Indirect Coombs Test. If there are no antibodies there is no Rh alloimmunization. Once sensitization occurs, Rh immune globulin is no longer effective. First-time sensitized pregnancies are followed with serial maternal titers. Maternal antibody titers cannot be used to screen for fetal anemia in affected pregnancies but only suggest severity of the HDFN. If antibodies are present in critical levels then various degree of fetal affection will occur if fetus is Rh-positive but will escape fetal anemia if the fetus is Rh D negative.
About 5% of the Indian population is Rh D negative. When prophylactic anti-D immunoglobulin was not available, the incidence of Rh D immunization was reported by the Institute of Immunohematology as 4 to 5% among Rh D negative women registered in the outpatient department of a tertiary care hospital at Mumbai (2).
History
In 1941, Levine et al, demonstrated that the maternal Rh antibodies were responsible for causing erythroblastosis fetalis (3). Soon, after this exchange transfusion was shown to improve the prognosis for the affected neonate. A study by Bevis showed that the severity of the fetal hemolytic disease could be predicted by serial amniocentesis (4). A major therapeutic breakthrough came in 1963 when Sir William Liley successfully performed the first intra-uterine fetal blood transfusion in severely affected fetus by intraperitoneal route using adult blood cells with fluoroscopic control (5). At about the same time several attempts were made to perform intravascular fetal blood transfusion by hysterotomy with very high maternal and fetal risks. Twenty years later, Rodeck et al reported a percutaneous technique for intravascular fetal blood transfusion using fetoscopy (6). Daffos et al introduced fetal blood sampling in 1983 under ultrasound guidance that has now become a routine procedure and guided the preferred technique for fetal blood transfusion (7). The development of anti-D immunoglobulin in 1961 fortunately avoids most Rh-negative women with such fetal intervention (8).
Genetics
The Rh blood group antigens are present as distinct trans-membrane proteins on the red blood cell (RBC) surface (9). Fischer and Race first proposed the concept of three genes that encode for the three major Rh antigen groups - D, C/c and E/e in 1946 (10). The Rh gene locus on short arm of chromosome one at 1p34 - p36 locus was discovered 45 years later by Cherif and Zahar in 1991 (11). The Rh gene consists of two closely linked and homologus genes with 96% similarity with each of 10 exons in length (12). One gene is designated as RhCcEe that encodes both Cc and Ee proteins. The second gene Rh D encodes the major antigen Rh D. An Rh D pseudogene has also been described (13) in which all the exons of the Rh D gene are present but the translation of the gene into a messenger RNA does not occur due to presence of stop codon. Since no Rh D protein is synthesized, the individual is serologically Rh D negative. The presence of such scenario in Rh D negative patient has implications in prenatal diagnosis of fetal blood group by polymerase chain reaction (PCR) on small amounts of fetal DNA obtained by amniotic fluid or chorionic villus sampling. Finally the serological Rh D negative means the absence of the major D antigen on the red cell surface either as a result of homozygous absence of Rh D gene or partial deletion of exons 7 to 9 or a stop codon exon 5 of the Rh D gene (14).
There are as many as 40 other Rh antigens but the Rh D epitope is the most immunogenic of the Rh system, followed by Rhc that is more than 20 folds less potent. The proportion of people who are Rh D negative varies according to race. The highest Rh D negative individuals are found in the Basque population from Spain which is as high as 30%. Dr Ian Donald observed that in China, Rh D negative population is most uncommon and suggested that the older civilization of Chinese has by process of natural selection bred out the undesirable gene. In China and Japan it is rare to find Rh D negative individual as the incidence drops to less than 1%. However, about 15% Caucasian population is Rh D negative compared to Afro-Caribbean black population who are 7 - 8% Rh D negative.
Pathophysiology
Red cell membrane contains numerous surface antigens including those of ABO and Rh groups. Maternal IgG antibodies can be generated against most of these antigens following exposure to an adequate quantity of red cell antigen due to transplacental fetomaternal hemorrhage or after heterologus blood transfusion. It has been demonstrated that around 75% women have fetal red cells in their circulation of variable numbers depending on period of gestation (15). For practical purposes anti-Rh D, anti-c, anti-E and anti-Kell antibodies have potential to cause moderate to severe HDFN (16, 17). Initial exposure to foreign red blood cell antigen leads to a primary immune response in the mother producing IgM antibody after a latent period of few weeks. After the D antigenic stimulus, IgM anti-D antibodies having the molecular weight of 90,000 daltons appears first. These IgM antibodies cannot cross the placenta and the fetus remain unaffected. On exposure of the mother to similar antigenic red cells during subsequent pregnancy the previously primed memory B cells produce IgG antibody. A considerably less antigenic stimulus is required for this secondary response compared to the primary exposure and much higher antibody titers are produced. These Rh IgG antibodies have molecular weight of 160,000 daltons which are able to cross the placental barrier via receptors for the IgFc portion located on synciotrophoblast and coat Rh D positive fetal red cells thus causing their sequestration. IgG sensitized red cells bind via the Fc part of the antibody molecule to FcY receptor (FcYR) on the mononuclear phagocytes in the fetal reticuloendothelial system and thus triggers its cytolysis (18). FcYR mediated phagocytosis by monocytes and macrophages are well developed by early second trimester at 17-18 weeks (19). Sensitized mothers carrying Rh D positive anemic fetus demonstrate high levels of IgG1 antibodies compared to control pregnancies. In addition, there is preferred accumulation of IgG1 in those fetuses affected by hemolytic process along with low levels of fetal IgG3 than are found in fetuses not at risk of HDFN. The IgG3 subclass antibody has a higher potential for inducing phagocytosis and monocyte adherence in-vitro than IgG1. However, IgG1 has a greater influence on the severity of HDFN in-vivo than IgG3 (20).
In the absence of anti-D prophylaxis programme, approximately 1% of Rh D negative women will have detectable anti-D antibodies in their serum by the end of their first pregnancy with Rh D positive fetus. A further 7 - 9% of these women will have detectable antibodies by six months following delivery and roughly the same number will develop antibodies during the second pregnancy with Rh D positive fetus. Thus around 17% of Rh D negative women get alloimmunized by a single Rh D positive fetus (21). The risk of Rh D alloimmunization during pregnancy increases with advancing gestation due to increase incidence of fetomaternal transplacental hemorrhage (15).
All Rh D negative women do not get alloimmunized with Rh D positive fetus in absence of anti-D prophylaxis. Some Rh D negative women are poor responders to Rh D antigen while others may produce anti-D IgG of low potency and efficacy at mediating FcY receptor interaction with phagocytic cells. After maternal alloimmunization to Rh D antigen it is estimated that only 50% of the affected fetus will have mild to no anemia, 25 to 30% will have moderate anemia that will require treatment in the neonatal period and the rest 20 - 25% will develop severe anemia and hydrops. These severely anemic fetuses likely have intrauterine fetal demise or neonatal death unless antenatal fetal therapy is initiated at a specialized center (22).
Hydrops Fetalis
Ascitis (Fig. 1), placentomegaly (Fig. 2), pericardial effusion and subcutaneous oedema (Fig. 3) are the classical features of fetal hydrops on ultrasonography and can also be seen in the neonate if delivered with hydrops (Fig. 4). The affected fetus becomes progressively anemic due to red cell hemolysis and compensates with cardiovascular adjustments and increasing hematopoeisis. Initially to maintain tissue oxygenation cardiac output is increased rather than fetal heart rate due to decrease in blood viscosity and fall in peripheral resistance secondary to vasodilation. In middle cerebral artery mean blood flow velocities due to fetal hypoxemia and anemia have hyperdynamic circulation primarily as a consequence of decreased blood viscosity (23).
Fetal hemoglobin concentration rises with gestational age and hydrops tends to occur at higher hemoglobin levels at later gestation compared to early gestational age (24). It is, therefore more appropriate to relate severity of fetal disease to the hemoglobin deficit for the gestational age. The presence of hydrops indicates fetal hemoglobin deficit of more than 7 g/dl for the gestational age. This means that fetal hemoglobin in the second trimester is less than 4g/dl or hematocrit less than 15%. However, at these low levels of hemoglobin fetus may not become hydropic. There are several possible explanations for the genesis of hydrops at this degree of anemia like cardiac failure due to myocardial dysfunction caused by fetal metabolic lactacidosis and increased capillary permeability caused by chronic tissue hypoxia (25). In addition iron overload resulting due to ongoing red blood cell hemolysis leads to free iron radical formation and contribute to endothelial dysfunction. Hypervolemia is also considered a reason for fetal hydrops related to a high output cardiac failure that can cause extravasation of fluids in tissues (26). Very high levels of Atrial Natriuretic Peptide are found in such hydropic fetuses implying expanded intravascular volume. Increased amniotic fluid volume appears before the onset of hydrops as normoxemic severely anemic fetuses is able to compensate by an increase in urine production (27).

- Gross ascitis in severely hydropic fetus in transverse section seen with floating bowel loops.

- Placentomegaly in fetus with gross fetal hydrops

- Scalp edema on ultrasound seen between the arrows in severe hydrops popularly known as ‘Buddha Sign’ on X-ray of such fetuses

- Hydropic baby being nursed in neonatal intensive care unit. The two-day old baby has gross ascitis with secondary hydrocele clearly seen in this picture. The ascitic fluid is being drained by continuous peritoneal drain placed in-situ.
Routine Rh Screening and Obstetric History
A blood sample must be collected from every woman at her first antenatal visit for ABO and Rh typing for any order of pregnancy. Rh D negative women and those having partial or weak ‘D’ antigens, were screened for the presence of anti-D in their serum by the indirect antiglobulin (Coombs) test. Paternal blood group and Rh testing should be the integral part of screening. The reduction of maternal surveillance after the father of the baby proves to be Rh-negative is useful unless there is a doubt regarding paternity. In the Rh-negative non- sensitized mother the indirect antiglobulin test should be repeated every four weeks and thereafter till delivery. In Rh alloimmunized mother Rh antibody titer and quantization are the tests employed for estimation of Rh antibodies in maternal serum. A rise in Rh antibody titer indicates Rh D positive fetus.
Screening for antibodies in the second half of the non-alloimmunized pregnancy is very important because it is the most likely time for Rh immunization secondary to transplacental hemorrhage. If in a non-alloimmunized pregnancy antepartum Rh-immune prophylaxis is administered at 28 weeks, a single anti-D titre at 35 weeks is helpful. A titre of Rh-antiglobulin in dilution of 1:8 or greater suggests active immunization (28).
Rh Titer
Several techniques are available to detect Rh antibody titer in maternal serum. The most reliable and sensitive technique is by Indirect Antiglobulin Test (Indirect Coombs Test) which is based on detection of IgG anti-D molecules of maternal serum on ‘O’ Rh D positive red cells, using a broad spectrum anti-human globulin (AHG) reagent. This test is method of choice in most of the centers for prediction of severity of Rh disease.
The detection of antibody necessitates determining the strength of the immunization by an antibody titer, which is estimated by serial dilutions. The degree of immunization corresponds well to the severity of fetal affection. However, a problem has developed with antibody titers today. Rh immunization has become so infrequent that some laboratories are no longer proficient in the technique. The laboratories thus capable of estimating accurate and reproducible titers play a very useful role in the management of the Rh alloimmunized patients. Laboratories have a critical titer at which there is a significant risk of fetal hydrops mostly between 1:8 and 1:32 dilutions. The absence of antibodies on the initial visit in the patient and subsequent detection on follow-up, groups this patient into the first immunized pregnancy. The alloimmunization of a patient by a prior pregnancy, groups this patient into the subsequent immunized pregnancy. Fetal death and hydrops due to Rh alloimmunization occurs very rarely before 18 weeks. If hydrops has occurred in one pregnancy, then it is likely to re-occur in subsequent pregnancies carrying an Rh-incompatible fetus if treatment by fetal blood transfusion is not initiated (29).
Rh Typing of Fetus
Prenatal DNA based Rh typing of the fetus could show positive results as early as 9 weeks of gestation by obtaining trophoblasts from endocervical canal. Amniotic fluid cells are also be used for this purpose obtained from amniocentesis undertaken as early as 15 weeks. Efforts are now being made to obtain DNA from fetal cells in the maternal peripheral circulation to establish a non-invasive technique that will be available for clinical use in near future (30).
Factors Influencing Rh D Immunization
Besides the use of anti-D IgG several other factors determine the severity of the Rh disease. The D antigenic response is necessary for stimulation of anti-D production. Since D antigen exists only on human RBCs, exposure of Rh D positive blood cells is a prerequisite for sensitization. Rh(D) incompatible transfusion and Rh D incompatible pregnancy are the main sources of D antigen stimulation (31).
Fetomaternal hemorrhage (FMH) is common in third trimester (15) and occurs in about 7% of pregnant women. Complicated and instrumental deliveries increase the risk of FMH. Rh(D) antigen is present on the RBC of a 38 days old fetus. Therefore, the FMH in first trimester spontaneous abortion and induced abortion warrants anti-D IgG prophylaxis. Studies have shown that 99.2-99.3% of women have a FMH less than 4ml at delivery. Up to 50% of larger FMHs occur after normal deliveries (32). However, the following clinical circumstances are more likely to be associated with large FMH:
traumatic deliveries including caesarean section
manual removal of the placenta
stillbirths and intrauterine deaths
abdominal trauma during the third trimester
twin pregnancies (at delivery)
unexplained hydrops fetalis
Tests to estimate the size of the FMH are recommended in many countries including the UK, the USA, Canada, France and Ireland, although not in most European countries. Acid elution technique which detects fetal haemoglobin (HbF) to measure FMH was reported by Kleihauer et al (33). It has been modified by Nierhaus and Betke (34). A thin blood smear prepared from maternal blood collected with ethylenediamine tetra acetic acid (ETDA) is treated with acid hematoxylin to elute maternal hemoglobin and to stain lymphocytes, which may otherwise be identified as fetal cells. The smear is then counterstained by erythrocin to stain fetal red cells. Under light microscope, the number of fetal cells per 10,000 maternal ghost cells is counted. The amount of fetal blood in maternal circulation is calculated by this relationship. The presence of upto four fetal RBC per 10,000 maternal cells signifies 0.15 ml of FMH.
In some European countries (exceptions include the UK, France and Ireland) a standard postnatal dose of 200 µ,g - 300 µ,g (1000-1500 IU) is used with no requirement for a routine Kleihauer test (35). In India since the standard dose of anti-D Ig is 300 µ,g (1500 IU) that is sufficient to clear about 15 ml of FMH, so routine Kleihauer test is not advised. Unfortunately, this policy does not take account of the fact that up to 0.3% of women have a FMH greater than 15ml that will not be covered by 300 µ,g (1500 IU) of anti-D Ig. The test is also required when massive FMH is suspected. In UK if the 1500 IU dose is implemented without a test to quantitate FMH, over 200 women each year will receive less protection than they do now (36). The recommended policy in the UK is to obtain an anticoagulated blood sample as soon as possible (within two hours) after delivery and to undertake a Kleihauer screening test to identify women with a large FMH who need additional anti-D Ig.
While the Kleihauer acid elution test is the test usually undertaken in the UK and Canada, tests that specifically identify Rh D positive red cells are used in the USA. Flow cytometry offers an alternative technique for quantifying the size of FMH (37). It has a number of advantages in that results are more accurate and more reproducible than those from the Kleihauer test and that it detects Rh D positive cells, making it particularly helpful in patients with high HbF levels. Not all hospitals will have ready access to a flow cytometer though several Blood Centres offer to estimate FMH. Flow cytometry is probably most effectively employed in those cases where a Kleihauer screening test indicates a large FMH that requires accurate quantitation and follow-up. The direct flow cytometry method uses fluorescent isothiocynate (FITC) or phycoerythrin (PE) labeled monoclonal anti-D. In the indirect method anti-human globulin reagent labeled with FITC or PE is employed. Chapman et al (38) has recommended flow cytometric technique for confirmation of amount of FMH when the leak is greater than 4 ml. The rosetting technique1 is a relatively simple serological method that offers another alternative for quantifying FMH of Rh(D) positive red cells greater than 4ml.
Effect of ABO Incompatibility
An ABO incompatible pregnancy offers protection against Rh alloimmunization, as A or B group fetal red cells are destroyed by anti-A or anti-B antibodies in the maternal circulation before Rh D can be recognized by the maternal immune system. Finn et al found that these feto-maternal microtransfusions were much less likely to occur in the case of women who were bearing an ABO incompatible baby (8). This reduces the chance for maternal sensitization by 20% (39). However, vice versa, alloimmunization capability is enhanced if the Rh positive fetus is ABO compatible with Rh D negative mother due to delayed destruction of fetal red cells by maternal immune system.
Immune Response of D antigen
It is surprising to note that iso-immunization does not occur in every case which an Rh D negative woman bears an Rh D positive child, but the phenomenon is doubtless dependent on the size, quantity or repetition of the antigenic stimulus. Once the mother has become immunized, her serum will contain antibodies for most of the rest of her life. Immune response to D antigen is genetically controlled. About one third Rh D negative women do respond to D antigen. The remaining two third women are either good or poor responders. Poor responders require several D antigenic stimuli before they produce anti-D Ig. The immunogenicity also depends on the number of D antigenic sites per RBC (31). It follows therefore that if iso-immunization has occurred as a result of pregnancy, the results are more likely to appear, not in that pregnancy but in the subsequent ones on disruption of placental continuity that provokes sensitization in susceptible women by secondary immune response.
Rh Status of Father
All children of Rh D negative mothers will be Rh D positive if the father is homozygous Rh D positive (D/D) but 50% children will be Rh D negative if the father is heterozygous (D/-) positive. If both the parents are Rh D negative, then the children will always be Rh D negative (40). Determination of definite Rh genotype is difficult, as anti-D antibody does not exist. With the help of anti-C, anti-c, anti-D, anti-E and anti-E reagents, the Rh phenotype can easily be identified.
Other Prenatal Investigations
Ultrasound assessment of at-risk fetus is mandatory for detecting fetal hydrops. Weekly assessment of high-risk patients is appropriate. The sonographic features of hydrops fetalis include ascites, pericardial and pleural effusion, subcutaneous and scalp edema, polyhydramnios and placentomegaly. However, only two-thirds of fetuses with such low hemoglobin demonstrate fetal ascitis. The earliest sonographic feature of hemolytic disease has been said to be enlargement of the heart and in particular reference to the right atrium (29).
Doppler studies in the evaluation of severe alloimmunization are performed in the view of correlation between decrease in fetal hemoglobin and increased maximum systolic velocities in various fetal blood vessels as a consequence to decrease in blood viscosity and increase in cardiac output. One of the most significant breakthroughs in recent years has been the research that validates the Peak Systolic Velocity Middle Cerebral Artery (MCA-PSV) as a reliable screening tool to detect fetal anemia (Fig. 5). Vyas and coworkers (1990) were the first to report the use of the Doppler velocity in the MCA to detect fetal anemia (41). This method is based on the fact that anemic fetuses have an increased blood flow velocity due to high hemodynamic circulation. Mari and co-workers (2000) generated the normative data for gestational age using threshold value of 1.5 Multiples of Median (MoM) for MCA-PSV to predict moderate to severe anemia. Doppler measurement of the MCA-PSV is performed (42) in recumbent position. The fetal vertex is visualized, and an axial plane that included the thalami and cavum septum pellucidum is obtained. The transducer is moved caudally until the circle of Willis is in view with color flow Doppler imaging. The MCA closest to the maternal skin is identified, and the angle of the ultrasound beam to the MCA blood flow is positioned as close to zero degrees as possible. The velocity measurement is made as close to the MCA origin from the circle of Willis as possible. MCA-PSV measurements are taken during periods of fetal quiescence (absent fetal breathing motion or fetal movements). The peak of the velocity waveform is measured. Multiple measurements are obtained during each ultrasound examination. The highest value, obtained with an angle of insonation as close to zero as possible, is recorded as the PSV measurement. If the MCA-PSV measures greater than 1.5 MoMs for gestational age, then the pregnancy is considered to be at risk of significant fetal anemia (Fig. 6). It has a positive predictive rate for moderate to severe fetal anemia of 74% with a 10% false positive rate. MCA Dopplers can be started as early as 15 weeks gestation but are not reliable after 35 weeks. The study of MCA-PSV is initiated whenever the antibody Coombs' titre rises beyond the critical value while on regular antenatal follow-up. The advantage of serial MCA measurements is that they reduce the need for invasive diagnostic procedures like amniocentesis and cordocentesis by more than 70% (42). Doppler measurement of the MCA- PCV can safely replace invasive testing in the management of Rh-alloimmunized pregnancies (43).

- Middle cerebral artery-peak systolic velocity measurement at origin from circle of Willis.
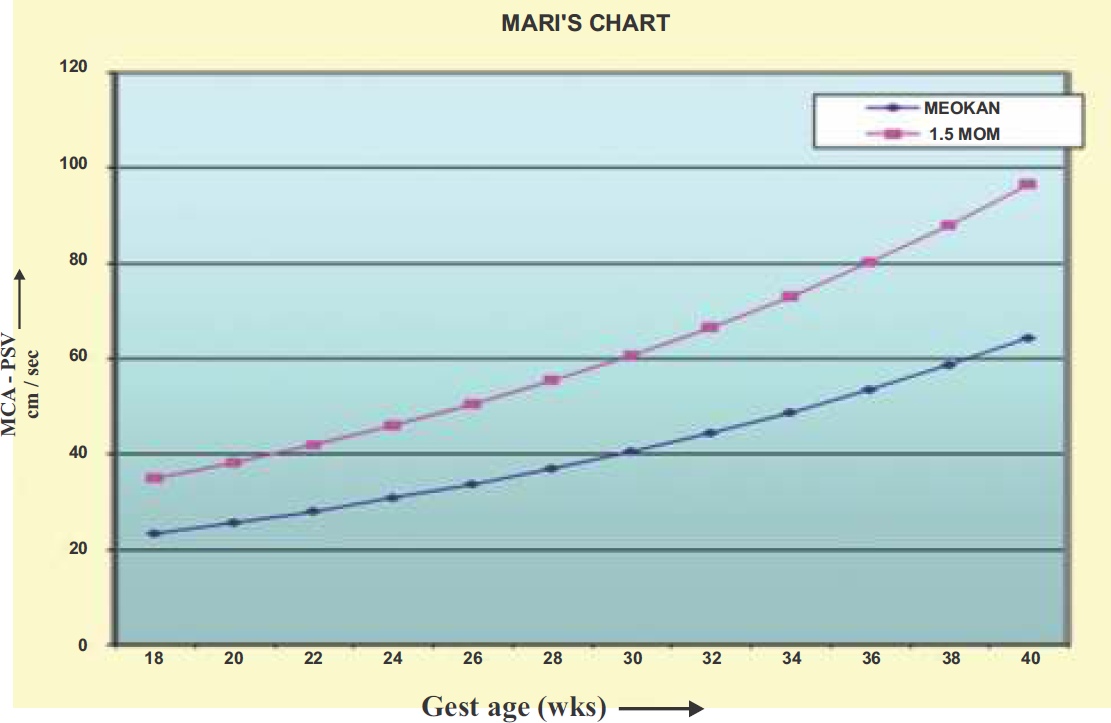
- The Mari et al (42) chart developed to show an association between Middle Cerebral Artery-Peak Systolic Velocity in reference to gestational age. The graph line marked with filled rectangles marks the cut-off value in 1.5 MoMs of MCA-PSV for the gestational age.
Invasive Testing of the Fetus
Pregnancies complicated by Rh alloimmunization have been evaluated to assess degree of fetal anemia indirectly with the use of serial invasive amniocentesis to determine bilirubin levels as a result of fetal hemolysis by measuring in the amniotic fluid, the change in optical density at a wavelength of 450 nm (AOD450) by spectrophotometric analysis; however, this procedure carries risks. Nicolaides et al have shown that the backward extrapolation of the Liley's chart lines is of little value in determining severity of fetal anemia before 27 weeks gestation. The wide scatter and fluctuation of OD450 values leads to a 68% false negative rate for severely affected fetuses between 18 and 25 weeks gestation. Doppler ultrasonography of middle cerebral artery has emerged as more sensitive and more accurate (95 percent confidence interval) than measurement of amniotic-fluid OD (29). The other method of assessing the degree of fetal anemia is directly by hematological studies on fetal blood sample. The indication for the invasive testing should be decided on:
Previous obstetric history
Duration of pregnancy
Maternal antibody levels
Ultrasonography
Doppler studies with MCA-PSV >1.5 MoMs for the gestational age
The aim of management in Rh alloimmunized pregnancy is to allow the pregnancy to continue to a safe gestational age, ideally 37 completed weeks or more. The fetus at the time of delivery should not be hydropic or severely anemic for a favourable outcome. This specialized management requires expertise and referral to a fetal medicine centre once invasive testing is being contemplated. In the present scenario there has been a decline in number of amniocentesis for the favour of non-invasive assessment and fetal blood sampling (43).
Cordocentesis was introduced in the mid 1980s. The direct access to the umbilical cord vessels by ultrasound guided needle puncture allows clinicians to measure fetal hematocrit, reticulocyte count, bilirubin level and a direct Coombs test. However, cordocentesis as a primary surveillance tool is not recommended due to 1% risk of fetal loss and chance of fetomaternal hemorrhage enhance maternal sensitization. Presently, cordocentesis is reserved as a second line diagnostic tool once amniocentesis or MCA Doppler suggests fetal anemia. Conventionally, serial amniocenteses were performed for spectrophotometric studies to detect fetal bilirubin by OD450 for estimating fetal anemia based on the Liley's reference chart. Serial peak middle cerebral artery velocities using Doppler ultrasound have now become the mainstay in these pregnancies to screen for fetal anemia (42). In anemic fetuses remote from term, intrauterine fetal blood transfusion is usually necessary either intravenously, through ultrasound-directed puncture of the umbilical vein with the direct intravascular or intra-peritoneal injection of red cells. In intra-peritoneal transfusion the transfused blood is deposited in the fetal peritoneal cavity. Perinatal survival rates of more than 90% have been reported in fetuses thus managed. Development of hydrops fetalis, however, has been reported to reduce the chance for a favourable outcome by up to 25%. Long-term studies have revealed normal neurological outcomes in more than 90% of such anemic fetuses treated with fetal blood transfusion.
Fetal Blood Transfusion
Delivery before 34 weeks in a severly anemic fetus is so readily rewarded by a neonatal death in olden days that every attempt was made to secure degree of maturity but most severely affected babies died in-utero often prematurely. In order to stave off intra-uterine death, intrauterine fetal transfusion (IUT) was introduced by Dr Liley in 1963 that provided hope to salvage these desperate cases (5). This procedure captured the imagination of the whole obstetrical world as it represented the first successful attempt at directly correcting a fetal disorder before birth. The procedure of intra uterine transfusion has been refined over the years from intra-peritoneal to intravascular or intra-cardiac depending on the clinical scenario.
Serial ultrasound examinations for MCA-PSV measurement are performed every 1 to 3 weeks depending on the antibody titer. Evidence of evolving fetal hydrops is evaluated at each ultrasound examination. If the MCA-PSV measured greater than 1.5 MoMs for gestational age, then the pregnancies are considered to be at risk of significant fetal anemia and are offered fetal blood sampling (42). Intra-uterine fetal blood transfusion is performed whenever fetal hemoglobin is less than 9 g% or hematocrit is less than 30% for the fetuses with gestational age less than 34 weeks (44). If the gestational age is more than 34-35 weeks, delivery is considered in view of expected fetal lung maturity. However steroids for fetal lung maturity should be administered before 34 weeks on regular based protocol in view of contemplating early delivery secondary to complication by fetal intervention. The pregnancy can be prolonged upto 35-36 weeks in absence of fetal anemia as predictability of MCA-PSV for fetal anemia decreases after 35 weeks of gestation (42).
Fetal surveillance following fetal blood transfusion is done by monitoring fetal heart on cardiotocography for about one hour after the procedure. The fetal heart variability observed during this period is often poor due to effect of fetal paralysis (45). The mother is advised to monitor daily fetal movement count and attend to bi-weekly to weekly monitoring by ultrasonography to evaluate MCA-PSV and umbilical artery velocimetry until next fetal blood transfusion.
After-effect of Fetal Blood Transfusion
After intra-uterine transfusion the fetal circulation will contain adult red blood cells and usually after three transfusions the entire fetal circulation contains no fetal red blood cells. Fetal erythropoiesis gets fully suppressed and fetal blood group at birth is ‘O’ Rh D negative. It can take several weeks for complete resumption of the neonatal erythropoiesis and top-up blood transfusions may be necessary as the baby develops anemia with growth. Regular monthly hemoglobin of such babies is advocated upto 6 months of age. However in refractory babies with absence of erythropoiesis, erythropoietin may prove to be efficacious. However, such babies born after being treated with intra-uterine blood transfusion show normal growth and neurological development (46, 47). Rh alloimmunized fetuses show high serum ferritin levels and repeated intra-uterine transfusions are further associated with iron overload in such babies. It is recommended that serum ferritin levels are monitored in such babies and iron supplementation withheld until its levels are in normal range (48).
Intra-peritoneal Fetal Blood Transfusion (IPT)
Sir William Liley successfully pioneered this procedure more than 40 years ago. The principle of intra-uterine fetal blood transfusion was based upon the fact that the fetus very readily absorbs blood directly from the peritoneal cavity far more readily than adult. The post mortem evidence indicates that the blood is completely absorbed within ten days of the IPT or earlier. However in the present day practice intra vascular route has been advocated the treatment of choice. IPT relies on placing the donor red cells into the peritoneal cavity so that they are absorbed into the fetal circulation via subdiaphramatic lymphatics and thoracic duct. The presence of ascitis in hydropic fetus reduces the efficacy of this procedure and increases the intra-peritoneal pressure in fetal abdomen that compromises venous return to fetal heart and cause bradycardia. Intravascular fetal blood transfusion (IVT) is considerably more successful in reverting the hydrops and ensuring survival in the neonatal period (49). The volume of blood transfused in IPT is dependent on empirical formulas as pre- and post-transfusion hemoglobin values for calculating amount of blood to be transfused is not available. In a specific clinical scenario IPT is the method of choice if treatment becomes necessary at very early gestation of 18 weeks or under when direct access to fetal vasculature is difficult and hazardous.
Technique of IPT involves placement of 18-20 gauge spinal needle into the fetal abdomen under ultrasound guidance. Ideally, the needle should enter the fetal abdominal cavity through the anterior abdominal wall, below the umbilical vein and above the fetal urinary bladder. This avoids trauma to the fetal liver and other fetal intra-abdominal organs. To verify that the needle is correctly placed the operator should either aspirate the ascitic fluid in hydropic fetus or in absence of ascitis, can infuse bolus of saline solution in the peritoneal cavity while observing the tip of the needle sonographically for confirming correct placement. Dr. Liley during his initial fetal transfusion via peritoneal route used to perform under fluoroscopic control (5). He used to confirm the correct needle placement by injecting radio-opaque dye in the fetal abdomen while observing fetal bowel loops displacement under fluroscopic control (Personal communication to author from Prof. Anjanellu). The needle can then be connected via three-way to the infusing tubing of closed circuit as described for IVT. Throughout the transfusion the tip of the needle is monitored sonographically to observe the flow of blood entering the peritoneal cavity and the fetal heart rate observed. The appearance of persistent bradycardia indicates that the IPT should be discontinued (29). The amount of donor blood to be given is calculated using the following empirical formula that calculates according to the gestational age of the fetus rather than the degree of anemia:
Volume of Donor Blood Required for Transfusion (ml)= (Gestation period in weeks - 20) X 10 ml
Intra Vascular Transfusion (IVT)
The direct IVT was introduced in mid 1980s. Till then IPT was the route of choice since Dr Liley introduced it in early 1960s. Experience in hydropic fetuses indicates that absorption from the peritoneal cavity is compromised. Harman et al divided fetuses into hydropic and non-hydropic group at time of first transfusion. He found a 13% increase in survival of non-hydropic fetuses using IVT as compared to IPT. In hydropic fetuses the rate of survival almost doubled with IVT. IVT also decreases the incidence of neonatal exchange transfusion and shortens the stay in neonatal intensive care unit (50). The IPT route still has a place to deliver red blood cells to the non-hydropic fetus when it is difficult to access the umbilical vein route either through the umbilical cord or the intra-hepatic part.
The blood used for fetal blood transfusion is adult group ‘O’ Rh D negative blood that should ideally be not more than 72 hours old and cross-matched with the maternal blood. It should be screened for hepatitis B and C, cytomegalovirus and HIV. To prevent graft- versus-host like complications in the fetus, the donor blood should be passed through leucocyte depletion filters followed by irradiation to remove the white blood cells. The donor blood cells are ideally packed to a hematocrit of 75 - 85% to minimize the volume of transfused blood.
Technique of intra-uterine fetal blood given by IVT involves insertion of a long spinal needle of 20-22 gauge (depending on period of gestation) under ultrasound guidance in the umbilical vein to obtain fetal blood sample followed by transfusion. The operator obtains the fetal venous access by a free hand technique or with needle guide attached with ultrasound transducer. Three trained persons are required for this procedure: an experienced operator who performs the cannulation of fetal umbilical vein and monitors the transfusion throughout by sonographic visualization, an assistant who administers blood and a third person assists in performing the blood tests and calculations. Tocolysis and suitable antibiotics may or may not be administered before this invasive procedure.
The umbilical vein at its placental insertion site is used for transfusion (Fig. 7 & 8). Frequently, placental position and fetal lie prevents safe access to the site of placental insertion of umbilical cord for transfusion. The alternative approach is to cannulate the umbilical vein in the free loop of umbilical cord or intra-hepatic part of umbilical vein that requires piercing the fetal abdomen with fetal paralysis with pancuronium. However, some workers do suggest universal fetal paralysis for the procedure by any vascular approach as fetal movements can be detrimental even after a safe vascular access (51). In my experience I have found that universal fetal paralysis is beneficial as any fetal movement may lacerate the vessel or dislodge the needle even if umbilical vein is approached in anterior placenta at insertion. Pancuronium is given to the fetus intra-muscularly or intra-vascularly (approximately 0.3 mg per kg of the estimated fetal weight) with ultrasonography guidance and has a theoretical advantage of increasing fetal heart rate secondary to catecholamine release. This helps in maintaining the fetal heart rate at a pre-transfusion rate and prevents fetal bradycardia during the procedure.

- Fetal blood transfusion in progress under ultrasound guidance with blood being transfused through a closed circuit with free hand technique.

- The umbilical vein is infused with concentrated donor red cells, producing turbulent cascade during infusion that stops immediately when the infusion stops. The thick arrow shows the needle tip and thin arrows show the turbulence inside the umbilical vein.
Once the access to the fetal circulation is obtained, one ml sample of fetal blood is drawn for determining fetal hematocrit and blood group. A fetal hematocrit less than 30% or below 2 SD for the gestational age, is considered an indication for in-utero transfusion at the same sitting (52). The volume of blood transfusion is determined by pre-transfusion fetal hematocrit, the estimated fetoplacental blood volume for the gestational age and the hematocrit of the donor blood. Nomograms have been worked out based on these parameters to provide an estimation of volume of donor blood required to raise the fetal hematocrit to 40% (53). The volume of red cell in milliliters can be calculated by Mandelbrot method using the formula given below (54):

At the end of the procedure 1 ml of fetal blood is aspirated to estimate post-transfusion fetal hematocrit. The blood is transfused at a rate of 10 ml per minute in 5-10 ml aliquots. During the procedure the flow of blood as a cascade is continuously visualized on the ultrasound screen that confirms correct needle placement. The fetal heart rate is periodically checked to look for fetal bradycardia. The onset of fetal bradycardia warrants abandoning of the procedure to prevent cardiac overload and arrest. A free-hand technique of fetal venous access allows independent movement of the ultrasound transducer for the periodic visualization of the fetal heart during the transfusion for monitoring. This free hand technique is preferred by most workers but requires high amount of expertise. However, the use of needle guide with the ultrasound probe has a better safety profile with less expertise.
Top up transfusions compared to exchange transfusions are quicker and reduce the risk of needle displacement, bacterial contamination and umbilical vein thrombosis. The second transfusion should not be performed later than 2 weeks after the first transfusion. In cases of severe fetal anemia and grossly hydropic fetuses or in cases where the first transfusion was small, the second transfusion may be required after a week. The mean fall of hematocrit is around 1% per day but it can have wide variation between the first and second transfusion. The fetal loss rate during the procedure ranges from 4 to 14%. The risk is more if fetal blood transfusion is given before 20 weeks period of gestation (29). Transient fetal bradycardia is the most common complication and it occurs in around 8% of the cases. The potentially fatal complications for the fetus are cord accidents like cord hematoma, umbilical artery spasm, hemorrhage from the cannulation site, thromboembolism and overloading of fetal circulation. Worsening degree of maternal s ensitization, chorioamnionitis, premature rupture of membranes and preterm labour are also possible complications of this invasive procedure (55). Graft-versus- host-reaction can take place if the transfused blood is not leukocyte depleted before transfusion.
Combined IVT and IPT
A combined IVT - IPT procedure results in a more stable fetal hematocrit between the two fetal blood transfusions (56). The hypothesis is that initially IVT is performed to achieve a final hematocrit of 40% followed by intraperitoneal infusion of donor blood which serves as a reservoir by allowing slow absorption of red cells between the procedures and allowing more stable hematocrit.
The technique used is the same for IVT. An adequate amount of donor blood is given intra-vascular to raise the fetal hematocrit to around 40%, as confirmed by a post transfusion hematocrit. The same spinal needle is then used to perform an IPT. This combined procedure becomes particularly easy if intra-hepatic part of umbilical vein is used for IVT (57). The amount of donor blood used in IPT should be the same that would be required intravascularly to raise the hematocrit to 60%. If a large amount of ascitic fluid is present in a hydropic baby then before IPT it should be removed.
Although the combined procedure has a theoretical advantage but most centers world over exclusively perform only IVT. The combined procedure warrants two needle punctures with a prolonged fetal procedure time that is considered as a disadvantage. The experience of fetal blood transfusions in twins is limited. There is a need to sample each fetus in dizygotic twins for antigen testing and follow-up with MCA Doppler studies. There is a difficulty in identifying corresponding cord insertions in twins that prevents transfusion to the corresponding anemic fetus. In this scenario intra-hepatic portion of umbilical vein may be the preferred target for vascular access. In monozygotic twins though the screening is done as of dizygotic twins but the fetal blood transfusion should be done with caution. The transfusion of one monochorionic twin may result movement of red blood cells to the other member of monochorionic pregnancy through intraplacental anastomosis. This may result in under or over transfusion of any of the twin (58).
Intra-cardiac Transfusion (ICT)
ICT is advocated in critical situations when severe early disease is present in fetuses. These fetuses when treated with IVT develop exsanguinations due to vessel trauma and presence of possible thrombocytopenia. In such situation the possibility of fetal resuscitation pass in a very short time. The fetal vascular collapse prevents repeating vascular puncture that leads to fetal demise. ICT in such fetuses is life saving. ICT also has an exceptional role of salvaging fetuses with very early hydrops especially less than 16 weeks gestation (59).
Technique of ICT involves use of 20-gauge spinal needle as it is easily maneuvered. The right ventricle of the heart is easily approachable but one can also use the left ventricle. IVT principals of fetal transfusion are followed as discussed. The turbulent cascade is visualized in the umbilical arteries exiting the fetus. The transfused blood should be warmed to normal body temperature before transfusion. Cold blood from the blood bank during ICT produces cardiac slowing and ventricular dysfunction. Although there is a potential risk for pericardial effusion by ICT but it is not common (60).
In my experience of more than 525 fetal transfusions have been performed till date at our center and the intravascular transfusions had been the mainstay of management with an excellent salvage rate of anemic fetuses. The IVT has been performed in a fetus of as early as gestational age of 18 weeks at our centre with a successful outcome. In one fetus of 17 weeks, IPT was performed, as the venous access was not feasible in such a small fetus followed by IVT at subsequent requirement with a success.
Red Cell Transfusion and Component Type
Red cells of O negative group used for IUT are centrifuged to increase the haematocrit to around 80%, leucodepleted and irradiated to prevent transfusion-associated graft-versus-host disease (TA-GvHD) and have specific features. These cells have only a 24-h shelf life following irradiation and the supplying Blood Bank ideally requires a minimum of 24 h notice.
Blood for IUT should not be transfused straight from 4°C storage due to risks of fetal bradycardia but there are no specifically designed warming systems for the small blood volume required and the component should not be exposed to radiant heaters or sunlight as the temperature is unmonitored and there is a risk of haemolysis. Transfusion volume required may be calculated based on donor and fetal haematocrit and the estimated fetoplacental blood volume. The fetoplacental volume depends on gestation age and fetal weight.
In urgent situations, if IUT units are unavailable, acceptable alternatives are irradiated neonatal red cell exchange units or irradiated pediatric packs. These should be available at all time in Blood Banks, so use of non-irradiated blood for IUTs should be extremely rare. In emergency situations where requesting irradiated red cells from the Blood Bank would cause life-threatening delay, it may be necessary to use a non-irradiated alternative, ideally a fresh neonatal pediatric pack (before the end of Day 5 following donation) or an exchange transfusion unit. The risk of TA-GvHD using these alternatives, although not eliminated, is acceptable in an emergency because these components have been leucodepleted and in most cases there will be no shared haplotype between donor and recipient. Maternal blood should not be used for IUTs because of the significant risk of TA-GvHD (61).
If the fetus receives more than three transfusions, fetal circulation will only show the transfused adult blood group ‘O’ Rh(D) negative. The fetal erythropoiesis is suppressed and can remain suppressed up to six months postnatally which should be monitored by regular hemoglobin estimation in the neonate. Top up transfusion may be necessary till resumption of erythropoiesis. These neonates do not have increased risk of compromise at birth and attain normal growth. There is also no neurodevelopment abnormality on long term follow-up (62). Other treatment modalities like maternal plasmapheresis or intravenous immunoglobulin (IVIG) administration has become history with the advent of modern fetal blood transfusion techniques. However, IVIG has a potential role in management of Rh alloimmunized neonate in the intensive care unit and also to the fetus while performing intrauterine blood transfusion (63).
Testing the Baby at Birth
As soon as the baby is born, 10 ml of cord blood should be collected in a heparinised tube for hemoglobin estimation, Coombs' testing and baseline bilirubin. The blood can be collected from the placental end of the severed cord but to avoid contamination with Wharton's jelly it should not be squeezed out. A blood smear is also made for detection of immature red cells. The hemoglobin level of the neonate at birth is an important prognostic indicator at birth.
Future Pregnancies
Once a woman has had one affected child by Rh allo-immunization then the outlook for further children is likely to be no better if not treated in-utero. It will also depend more on whether the male partner is homozygous or heterozygous. Unfortunately about three quarters of the fathers of affected children are in fact homozygous making outlook bleak for further child bearing as there is a tendency for the disease to be more severe in each successive instance. IUT, however may be impressive life saving procedure but how much better is to prevent iso-immunization in the first place that I feel should be the moto.
The advancement of performing fetal blood transfusion in the management of fetal disease caused by red cell alloimmunization has meant that sensitized women, who in past had suffered multiple fetal and neonatal losses, can now be optimistic about achieving a successful pregnancy outcome. Fetal blood transfusion is the mainstay of the management of Rh disease with MCA-PSV offering a reliable non-invasive modality for determining the fetal anemic status in such pregnancies. Doppler measurement of the peak velocity of systolic blood flow in the middle cerebral artery can safely replace invasive testing in the management of Rh-alloimmunized pregnancies (64, 65). It guides the timing of IUT, aids in monitoring the post transfusion period and helps in optimizing delivery timing to near term. These measures result in an improved perinatal outcome in pregnancies previously considered unsalvageable. Rh(D)-alloimmunized fetuses with ascites / hydrops at the time of the first transfusion have a survival rate of 87%. Alterations of several biochemical fetal blood indices are present at the first fetal blood sampling / transfusion, but most variables normalize with intravascular transfusions according to latest reports (66). The diagnostic accuracy of noninvasive fetal Rh determination using maternal peripheral blood is 94.8%. Its use can be applicable to Rh prophylaxis and to the management of Rh alloimmunized pregnancies in near future as a routine (67).
Red blood cell alloimmunization in pregnancy continues to occur despite the widespread use of both antenatal and postpartum rhesus immunoglobulin. It is due to inadvertent or inadequate omissions in administration as well as antenatal sensitization prior to rhesus immunoglobulin given at 28 weeks' gestation. The vigorous policy of Rh prophylaxis has reduced the Rh problem to about a tenth of what it was in Dr. Liley's time but it cannot eliminate altogether. Additional instances are attributable to the lack of immune globulins to other (non-D) red cell alloantibodies such as c, Kell and Fy. Since immune-prophylaxis is not available for non-rhesus-D disease, alloimmunization during pregnancy by these non-D red cell allo-antibodies will continue to occur!
References
- A retrospective review of isoimmunized pregnancies managed by middle cerebral artery peak systolic velocity. Am J Obstet Gynecol. 2004;190:1732-1736.
- [CrossRef] [PubMed] [Google Scholar]
- Changing pattern of Rh(D) immunization in Bombay. J Obstet Gynecol Indian. 1984;34:776-779.
- [Google Scholar]
- Isoimmunization in pregnancy: its possible bearing on the etiology of erythroblastosis foetalis. JAMA. 1941;116:825-827.
- [CrossRef] [Google Scholar]
- The antenatal prediction of the haemolytic disease of the newborn. Lancet. 1952;1:395-398.
- [CrossRef] [PubMed] [Google Scholar]
- Intrauterine transfusion of the fetus in hemolytic disease. Br Med J. 1963;2:1107-1109.
- [CrossRef] [PubMed] [Google Scholar]
- Direct intravascular fetal blood transfusion by fetoscopy in severe Rhesus isoimmunization. Lancet. 1981;1:625-627.
- [CrossRef] [PubMed] [Google Scholar]
- Fetal blood sampling during pregnancy with the use of a needle guided by ultrasound : a study of 606 consecutive cases. Am J Obstet Gynecol. 1985;153:655-660.
- [CrossRef] [PubMed] [Google Scholar]
- Experimental studies on the prevention of Rh haemolytic disease. Br Med J. 1961;1:1486-1490.
- [CrossRef] [PubMed] [Google Scholar]
- Two-dimensional iodopeptide mapping demonstrates erythrocyte Rh D, c and E polypeptides are structurally homologous but nonidentical. Blood. 1988;72:1424-1427.
- [CrossRef] [PubMed] [Google Scholar]
- Localization of the human Rh blood group gene structure to chromosome region 1p34.3 - 1p36.1 by in situ hybridization. Hum Genet. 1991;86:398-400.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic basis of the RhD-positive and RhD-negative blood group polymorphism as determined by Southern analysis. Blood. 1991;78:2747-2752.
- [CrossRef] [PubMed] [Google Scholar]
- The presence of an RhD pseudogene containing a 37 base pair duplication and a nonsense mutation in Africans with a Rh D-negative blood group phenotype. Blood. 2000;95:12-18.
- [CrossRef] [PubMed] [Google Scholar]
- Prenatal determination of fetal RhD type. N Engl J Med. 1994;344:205-206.
- [CrossRef] [PubMed] [Google Scholar]
- Fetomaternal transplacental hemorrhage during pregnancy and after delivery. Vox Sang. 1986;51:117-125.
- [CrossRef] [PubMed] [Google Scholar]
- Non- ABO clinically significant erythrocyte allo-antibodies in Caucasian obstetric patients. Transfusion. 1991;31:52S.
- [Google Scholar]
- Fetal anemia due to non- Rhesus-D red-cell alloimmunization. Semin Fetal Neonatal Med. 2008;13(4):207-214.
- [CrossRef] [PubMed] [Google Scholar]
- The ability of IgG subclasses to cause elimination of targets in vivo and to mediate their destruction by phagocytosis/cytolysis in vitro. In: Shakib F, ed. In: The Human IgG Subclasses. Oxford: Pentagon Press; 1990. p. :135-160.
- [CrossRef] [PubMed] [Google Scholar]
- A major role of class I FcY receptors in immunoglobulin G anti- D mediated red blood cell destruction by fetal mononuclear phagocytes. Obstet Gynecol. 1995;86:157-162.
- [CrossRef] [PubMed] [Google Scholar]
- Results of tests with different cellular bioassays in relation to severity of RhD haemolytic disease. Vox Sang. 1991;69:120-125.
- [Google Scholar]
- Blood Transfusion in Clinical Medicine (9th). Oxford: Blackwell Scientific Publications; 1993.
- A comparison of sheep and human fetal oxygen delivery systems with the use of mathematical model. Am J Obstet Gynecol. 1988;151:449-455.
- [CrossRef] [PubMed] [Google Scholar]
- Fetal haemoglobin measurement in the assessment of red cell isoimmunization. Lancet. 1988;1:1073-1075.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship of fetal hemoglobin and oxygen content to lactate concentration in Rh isoimmunized pregnancies. Obstet Gynecol. 1987;69:268-271.
- [Google Scholar]
- Iron overload, free radical damage, and rhesus haemolytic disease. Lancet. 1990;335:933-936.
- [CrossRef] [PubMed] [Google Scholar]
- Atrial natriuretic factor in human fetus: effect of volume expansion. J Pediatr. 1988;113:552-555.
- [CrossRef] [PubMed] [Google Scholar]
- Current management of the Rh-sensitized patient. Clinical Obstet Gynaecol. 1982;25(2):293-301.
- [CrossRef] [PubMed] [Google Scholar]
- Red cell alloimmunization. In: Fetal Medicine: Basic Science and Clinical Practice. Vol 59. (1st). London: Churchill Livingstone; 1999. p. :785-804.
- [Google Scholar]
- Prenatal genotyping of RHD and SRY using maternal blood. Vox Sang. 2003;85:300-306.
- [CrossRef] [PubMed] [Google Scholar]
- Laboratory techniques in evaluating a rhesus alloimmunized pregnancy, Chapter 4. In: Shah D, Salvi V, eds. In: The Rhesus Factor Current Concepts. New Delhi: FOGSI Publication Jaypee Brothers Medical Publishers; 2002. p. :41-57.
- [Google Scholar]
- Clinical high-risk designation does not predict excess fetal-maternal haemorrhage. Am J Obstet Gynecol. 1987;156:154-158.
- [CrossRef] [PubMed] [Google Scholar]
- Demonstration von fetalem hamoglobin in den Erythrocyten eines Blutausstriches. Klin Wschr. 1957;35:637-638.
- [CrossRef] [PubMed] [Google Scholar]
- quoted by Dacie SJV and Lewis SM (1984) In: Practical Haematology (6th). Edinburgh: Churchill Livingstone; 1968. p. :112.
- [Google Scholar]
- Committee for Proprietary Medicine Products. Notes for guidance: core summary of product characteristics for human anti-D immunoglobulin IM. 1994 III/34463/92- EN
- [Google Scholar]
- Royal College of Obstetricians & Gynaecologists and National Institute for Clinical Excellence's Technology Appraisal Guidance No 41. Guidance on the use of routine antenatal anti-D prophylaxis for RhD-negative women in pregnancy. 2002
- [Google Scholar]
- Flow cytometry in diagnosis and management of large fetomaternal haemorrhage. J Clin Path. 1995;48:1005-1008.
- [CrossRef] [PubMed] [Google Scholar]
- The estimation of fetomaternal haemorrhage. Transfusion Medicine. 1999;9:87-92.
- [CrossRef] [Google Scholar]
- The prevention and management of haemolytic disease of the newborn. J Roy Soc Med. 1994;87:256-258.
- [CrossRef] [PubMed] [Google Scholar]
- Blood Transfusion in Clinical Medicine (6th). Oxford: ELBS and Blackwell Scientific Publication; 1979. p. :292-352.
- Doppler examination of middle cerebral artery in anemic fetuses. Am J Obstet Gynecol. 1990;162:1066-1068.
- [CrossRef] [PubMed] [Google Scholar]
- Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. N Engl J Med. 2000;342:9-14.
- [CrossRef] [PubMed] [Google Scholar]
- Doppler ultrasonography versus amniocentesis to predict fetal anemia. NEngl J Med. 2006;355(2):156-164.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of anemia on fetal acid base status. Br J Obstet Gynaecol. 1987;94:880-883.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of neuromuscular blockage on human fetal heart rate and its variation. Br J Obstet Gynaecol. 1994;101:121-124.
- [CrossRef] [PubMed] [Google Scholar]
- Red blood cell alloimmunization in pregnancy. Semin Hematol. 2005;42(3):169-178.
- [CrossRef] [PubMed] [Google Scholar]
- Fetal growth and birthweight in isoimmunized pregnancies after intravenous intrauterine transfusion. Fetal Diagn Ther. 1993;8:407-411.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of intrauterine intravascular blood transfusion on iron metabolism in fetuses with Rh alloimmunization. Obstet Gynecol. 1991;77:558-562.
- [Google Scholar]
- Intrauterine transfusion-intraperitoneal versus intravascular approach: a case-control comparison. Am J Obstet Gynecol. 1990;162:1053-1059.
- [CrossRef] [PubMed] [Google Scholar]
- Hemolytic disease of the fetus and new born. In: Creasy RK, Resnick R, Iams J, eds. In: Maternal-fetal Medicine: Principles and Practice (5th). Philadelphia: WB Saunders; 2004. p. :537-562.
- [Google Scholar]
- Management of Rh-isoimmunized pregnancies: our experience. Med J Armed Forces India. 2007;63:7-11.
- [CrossRef] [PubMed] [Google Scholar]
- Normal values for human umbilical venous and amniotic fluid pressures and their alteration by fetal disease. Am J Obstet Gynecol. 1989;161:714-717.
- [CrossRef] [PubMed] [Google Scholar]
- Rh disease: intravascular fetal blood transfusion by cordocentesis. Fetal Ther. 1986;1:185-192.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of fetal blood volume for computer-assisted management of in- utero transfusion. Fetal Ther. 1988;3:60-66.
- [CrossRef] [PubMed] [Google Scholar]
- Management of fetal hemolytic disease by cordocentesis. Am J Obstet Gynecol. 1991;165:1302-1307.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of four types of intrauterine transfusion: effect on fetal hematocrit. Fetal Ther. 1989;4:126-137.
- [CrossRef] [PubMed] [Google Scholar]
- Fetal blood sampling from the intrahepatic vein: analysis of safety and clinical experience with 214 procedures. Obstet Gynecol. 1990;76:47-53.
- [Google Scholar]
- Management and outcome of fetomaternal Rh alloimmunization in twin pregnancies. Fetal Diag Ther. 1999;14:26-30.
- [CrossRef] [PubMed] [Google Scholar]
- Fetal intracardiac transfusions in patients with severe rhesusisoimmunization. Br Med J. 1988;296:885-886.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound in the management of the allo-immunized pregnancy. In: Fleischer AC, Manning FA, Jeanty P, Romero R, eds. In: Sonography in Obstetrics and Gynaecology [Principles and Practice] (6th). New York: McGraw Hill; 2001. p. :683-709.
- [Google Scholar]
- Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol. 2016;175:784-828.
- [CrossRef] [PubMed] [Google Scholar]
- Sensorineural outcome at two years for survivors of erythroblastosis treated with foetal intravascular transfusion. Obstet Gynecol. 1993;81:931-935.
- [Google Scholar]
- Fetal in travenous immunoglobulin therapy in rhesus hemolytic disease. Gynecol Obstet Invest. 2007;63(3):176-180.
- [CrossRef] [PubMed] [Google Scholar]
- Doppler ultrasonography versus amniocentesis to predict fetal anemia. N Engl J Med. 2006;355(2):156-164.
- [CrossRef] [PubMed] [Google Scholar]
- Use of peak systolic velocity of the middle cerebral artery in the management of fetal anemia due to fetomaternal erythrocyte alloimmunization. J Gynecol Obstet Biol Reprod(Paris). 2008;37(2):163-169.
- [Google Scholar]
- The effects of serial intravascular transfusions in ascitic/hydropic RhD-alloimmunized fetuses. Ultrasound Obstet Gynecol. 2005;25(2):144-148.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of noninvasive fetal Rh genotyping from maternal blood-a meta-analysis. Am J Obstet Gynecol. 2006;195(4):1163-1173.
- [CrossRef] [PubMed] [Google Scholar]





