Translate this page into:
Association of circulatory chemerin levels with the severity of pre-eclampsia: A systematic review and bootstrapped meta-analysis
*Corresponding author: Sadhana Sharma, Department of Biochemistry, All India Institute of Medical Sciences Patna, Bihar, India. drsadhanas@aiimspatna.org
-
Received: ,
Accepted: ,
How to cite this article: Varikasuvu SR, Madhuri M, Ali A, Gowtham K, Jegatheesan J, Ranjan A, et al. Association of circulatory chemerin levels with the severity of pre-eclampsia: A systematic review and bootstrapped meta-analysis. Ann Natl Acad Med Sci (India) 2024;60:261-6. doi: 10.25259/ANAMS_86_2024
Abstract
Introduction
This study presents a systematic review and meta-analysis to investigate the association between circulating chemerin levels and the severity of preeclampsia (PE).
Material and Methods
A thorough search was conducted across multiple databases, including PubMed/MEDLINE, SCOPUS, and the Cochrane Library, up to March 31, 2024, focusing on observational studies that compared chemerin levels in patients with severe versus mild PE. Two independent reviewers extracted mean and standard deviation (SD) values of chemerin levels from these studies. The quality and risk of bias of the included studies were assessed using the Newcastle–Ottawa Scale. Standardized mean differences (SMD) with 95% confidence intervals (CI) were calculated to evaluate the difference in chemerin levels between severe and mild PE groups. Sensitivity analyses were performed to address heterogeneity and ensure the reliability of the findings. Additionally, a bootstrapped meta-analysis with 1,000 and 10,000 simulations was conducted to further validate the results.
Results
Six observational studies were included in the analysis. The findings revealed that patients with severe PE had significantly higher chemerin levels compared to those with mild PE (SMD 1.55, 95% CI 0.67–2.43, p < 0.0001). The bootstrapped meta-analysis supported the accuracy of these findings (SMD = 1.54, CI = 0.74–2.38). No publication bias was identified using Begg’s and Egger’s tests, and the sensitivity analysis confirmed the robustness of the results.
Conclusion
This meta-analysis provides compelling evidence that chemerin levels are significantly elevated in patients with severe PE compared to those with mild PE. Further research is warranted to investigate the potential of circulating chemerin as a diagnostic marker for PE severity.
Keywords
Adipokine
Chemerin
Pre-eclampsia
Systematic review
Meta-analysis
INTRODUCTION
Complications related to pregnancy significantly increase mortality risk, especially in developing countries. Preeclampsia (PE), characterized by hypertension and proteinuria after 20 weeks of gestation, can manifest as either mild or severe. While mild PE includes symptoms like chest pain, vomiting, and high blood pressure, severe PE involves more serious conditions like abdominal pain, kidney failure, and blurred vision. PE is associated with liver and kidney dysfunction, pulmonary edema, thrombocytopenia, and a higher risk for diabetes and cardiovascular diseases. According to the World Health Organization, the global incidence of PE ranges from 2 to 10% of pregnancies, with a related mortality rate of 12%.1,2
The exact pathophysiology of PE is not fully understood, but it is believed to involve placental hypoxia, oxidative stress, and immune modulation, leading to the production of proinflammatory cytokines. Adipokines, such as chemerin, are gaining attention as potential early biomarkers for PE. Chemerin is involved in processes like adipogenesis, energy metabolism, and inflammation. Recent studies have suggested a link between chemerin levels and PE, but there is limited direct evidence on whether these levels correlate with PE severity.3–5
This systematic review and meta-analysis aim to provide precise information on the association between circulating chemerin levels and PE severity by comparing severe and mild PE groups. Sensitivity and bootstrapped meta-analyses were also conducted to ensure robust and precise outcomes.
MATERIAL AND METHODS
This review followed the PRISMA (Preferred Reporting Items For Systematic Reviews And Meta-Analysis) guidelines and was registered with PROSPERO (CRD42024526676).
Search strategy
We conducted a comprehensive literature search up to March 31, 2024, using PubMed, Cochrane Library, Scopus, and other databases. The search terms included “Chemerin” and “preeclampsia” without any time or language restrictions. Additional articles were identified through manual searches of bibliographies and contacting authors for unpublished data. Three researchers independently performed the literature searches. Any differences in their findings were resolved through discussions with other authors. Additionally, efforts were made to acquire any unpublished data. When necessary, the corresponding authors of the respective articles were contacted for further information.
Study selection criteria
To be included, studies had to be observational, focused on circulating chemerin levels in preeclampsia (PE), and compared levels between severe and mild PE groups. Participants needed to be human, with PE diagnosis criteria defined according to guidelines6 from the ‘American College of Obstetricians and Gynecologists (ACOG) or the International Society for the Study of Hypertension in Pregnancy (ISSHP). The studies also had to be written in English. Exclusion criteria included studies that did not specify whether PE was severe or mild, studies that only compared chemerin levels between PE and control groups, studies focusing on diseases other than PE, duplicate reports, reviews, experimental animal studies, letters to the editor, and commentaries.
Data extraction and quality assessment
After applying the inclusion criteria, the following details were extracted from each included study: first author names, country and year of publication, number of participants in severe and mild PE groups, PE diagnosis criteria, means, and standard deviations (SD) for age, anthropometric data, and circulating chemerin levels, as well as the chemerin measurement method and units. If data were reported in quartiles or as medians and ranges, conversions were performed to make them suitable for meta-analysis.7,8 Study quality was assessed using the Newcastle–Ottawa Scale (NOS) for observational studies, which scores studies from 0 to 9 based on selection, comparability, and exposure criteria.9
Statistical analysis
The meta-analysis aimed to compare chemerin levels between severe and mild preeclampsia (PE) groups using standardized mean difference (SMD) with a 95% confidence interval (CI) as the outcome measure. A random-effects model was applied to the data. Heterogeneity was assessed using the DerSimonian-Laird estimator, Q-test, and I2 statistic. If heterogeneity (tau2 > 0) was detected, a prediction interval for true outcomes was calculated. Potential outliers and influential studies were identified through studentized residuals and Cook’s distances, with outliers defined using Bonferroni correction and influential studies identified if Cook’s distance exceeded the median plus six times the interquartile range.
Funnel plot asymmetry was examined using the rank correlation and regression tests, with the ‘trim-and-fill method” applied to adjust for significant publication bias. The robustness of the meta-analysis was evaluated through a one-study leave-out sensitivity analysis. All analyses were two-tailed, with a significance threshold of p < 0.05. The primary analyses were performed using Review Manager (version 5.4), Jamovi (version 2.3), and OpenMeta(Analyst), while the bootstrapped meta-analysis was conducted using OpenMEE software.
RESULTS
Search results and study characteristics
The literature search identified 27 articles on chemerin levels in PCOS. After removing duplicates, reviews, and commentaries, 11 studies were selected for full-text review. Of these, five did not report chemerin levels between severe and mild PE patient groups, leaving six studies for the final analysis.10-15 The PRISMA flow diagram is shown in Figure 1.
- The study flowchart.
Study characteristics and Newcastle–Ottawa Scale quality scores are detailed in Supplementary Material [Table 1], with scores ranging from 6 to 8, indicating medium to high quality. All six studies measured serum chemerin using the ELISA method. PE diagnosis followed ACOG guidelines, with criteria including gestational blood pressure >140 mmHg systolic or >90 mmHg diastolic and proteinuria in previously normotensive patients before 20 weeks of gestation. Maternal age averaged between 25 and 34 years. Gestational age was reported in three studies as being in the third trimester,10,12,14 and in two studies as either second or third trimester,11,13 while one study sampled in the first trimester before PE diagnosis.15 The studies had either cross-sectional10-14 or prospective cohort designs.15
| Study, Year, Country | Severe PE | Mild PE | Sample, Method, Units | Confounder adjustment | NOS score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean age | Mean GA | Mean BMI | Chemerin (Mean) | Chemerin (SD) | n | Mean age | Mean GA | Mean BMI | Chemerin (Mean) | Chemerin (SD) | ||||
| Al-Refai AA.10 (2012), Saudi Arabia | 10 | 34.37 | 32.68 | 30.46 | 479 | 158.56 | 19 | 34.37 | 32.68 | 30.46 | 281.95 | 144.54 | Serum, ELISA, ng/mL | Age, BMI, GA | 8 |
| Cetin O et al.11, (2017), Turkey | 43 | 27.65 | NA | 26.10 | 394.72 | 100.01 | 45 | 28.11 | NA | 26.86 | 322.11 | 37.6 | Serum, ELISA, ng/mL | Age, GA | 7 |
| Duan DM et al.12, (2012), China | 23 | 29.19 | 35.83 | 23.34 | 289.6 | 74.43 | 49 | 29.19 | 35.83 | 23.24 | 228.1 | 87.99 | Serum, ELISA, ng/mL | Age, GA | 7 |
| Murad AM et al.13, (2020), Iraq | 33 | 24.45 | NA | 28.43 | 435.06 | 55.4 | 33 | 25.00 | NA | 26.61 | 227.49 | 57.4 | Serum, ELISA, ng/mL | Age, BMI, GA | 8 |
| Wang L et al.14, (2015), China | 30 | 28.5 | 37.4 | 28.65 | 493.83 | 105.23 | 30 | 27.9 | 38.5 | 27.49 | 330.23 | 56.22 | Serum, ELISA, ng/mL | Age, GA | 7 |
| Xu QL et al.15, (2014), China | 18 | 27.2 | 10.67 | 25.4 | 365.5 | 116.5 | 23 | 27.2 | 10.67 | 25.4 | 270.3 | 91.8 | Serum, ELISA, ng/mL | Age, GA | 7 |
BMI: Body mass index, GA: Gestational age, PE: Preeclampsia, NA: Not applicable, NOS: Newcastle-ottawa Scale, ELISA: Enzyme-linked immunoassay, SD: Standard deviation.
Circulating chemerin between severe and mild PE patients
Six studies comparing chemerin levels between 157 severe PE and 199 mild PE patients were included.10-15 The meta-analysis using a random-effects model estimated an average standardized mean difference of 1.55 (95% CI: 0.67–2.43), showing significantly higher chemerin levels in severe PE [z = 3.46, p = 0.0005, Figure 2]. The Q-test indicated heterogeneity among true outcomes (Q(5) = 46.38, p < 0.0001, tau2 = 1.0891, I2 = 91%). A 95% prediction interval for true outcomes ranged from –0.67 to 3.77, suggesting that while the average outcome is positive, some studies might report negative outcomes. One study13 was identified as a potential outlier with a studentized residual larger than ± 2.63 and was also considered overly influential according to Cook’s distances.
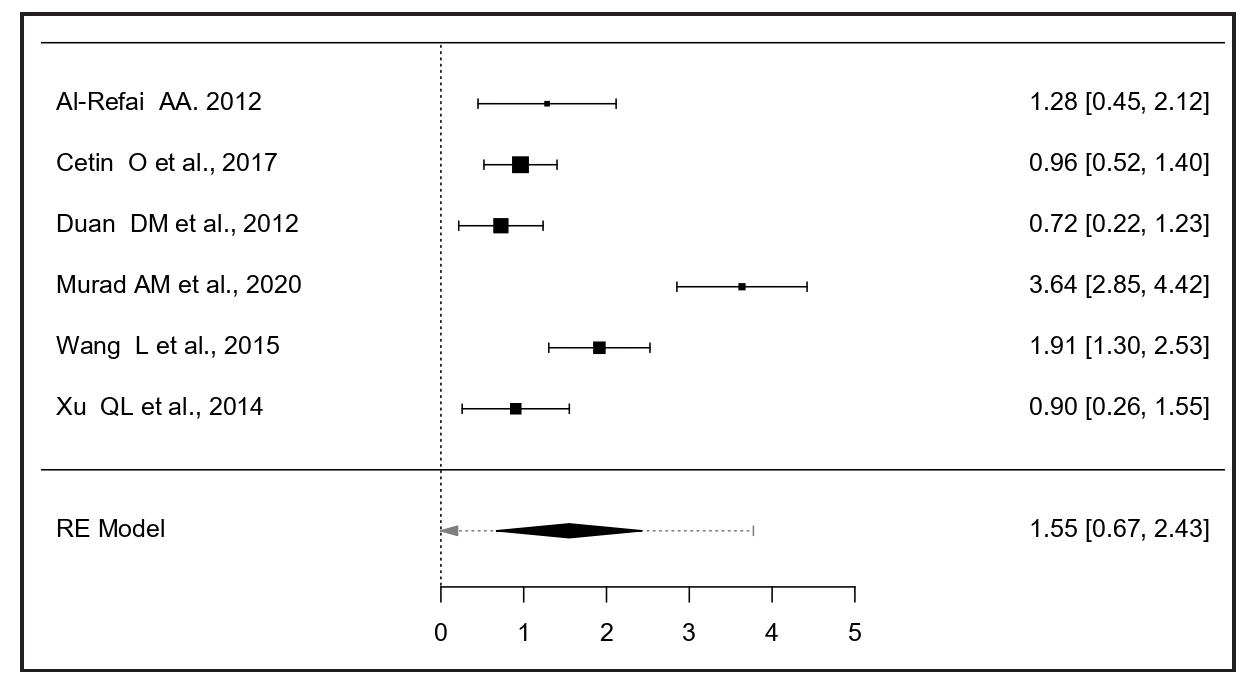
- The forest plot comparing serum chemerin between severe and mild PE patients. PE: Preeclampsia.
Publication bias and sensitivity analysis
As shown in Figure 3, there was no evidence of funnel plot asymmetry. Both the rank correlation and regression tests indicated no asymmetry (p = 0.27 and p = 0.18, respectively). A one-study leave-out sensitivity analysis confirmed the stability of the results, indicating consistently higher chemerin levels in severe PE regardless of which study was omitted [Figure 4].
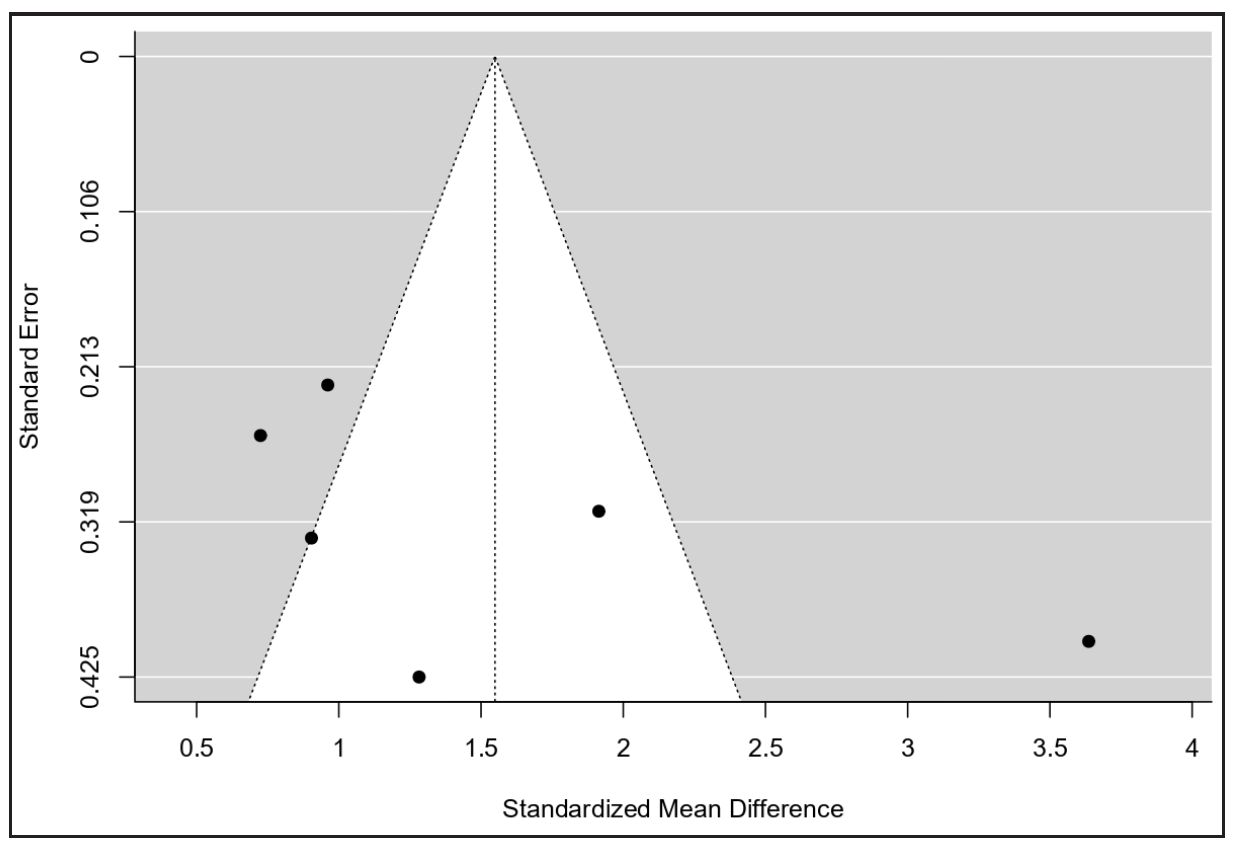
- The funnel plot for publication bias.
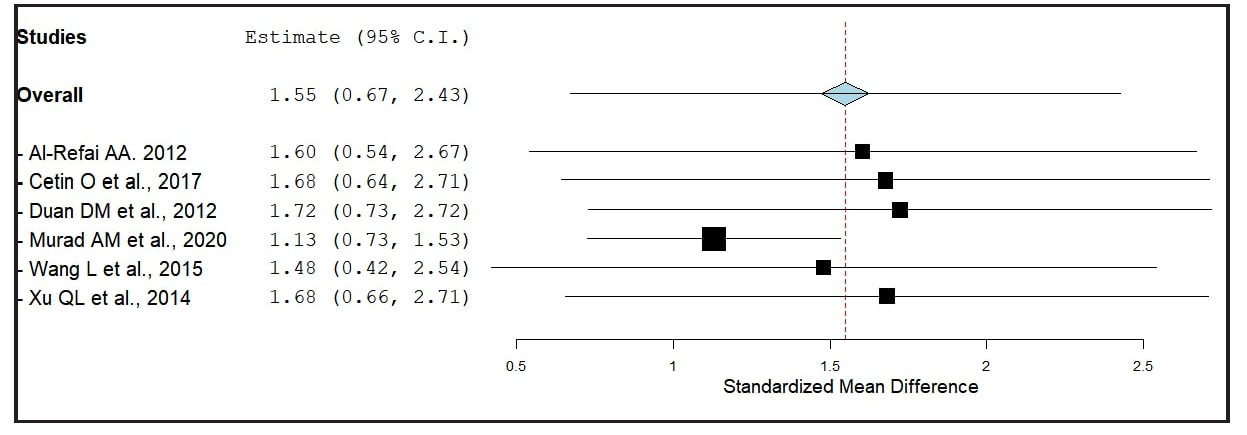
- The results of sensitivity analysis.
Bootstrapped meta-analysis
A bootstrapped meta-analysis with 1000 and 10,000 replicates [Figures 5 and 6] supported the accuracy of the original meta-analysis. The mean SMD over 1000 replicates was 1.54 (95% CI, 0.74–2.38), and over 10,000 replicates was 1.55 (95% CI, 0.74–2.36), as shown in Table 2. Bootstrapping is particularly valuable when dealing with a small sample size, an unknown population distribution, or a complex or non-standard statistics. It also helps assess the stability of results by repeatedly resampling from the original data to calculate confidence levels and other accuracy measures.
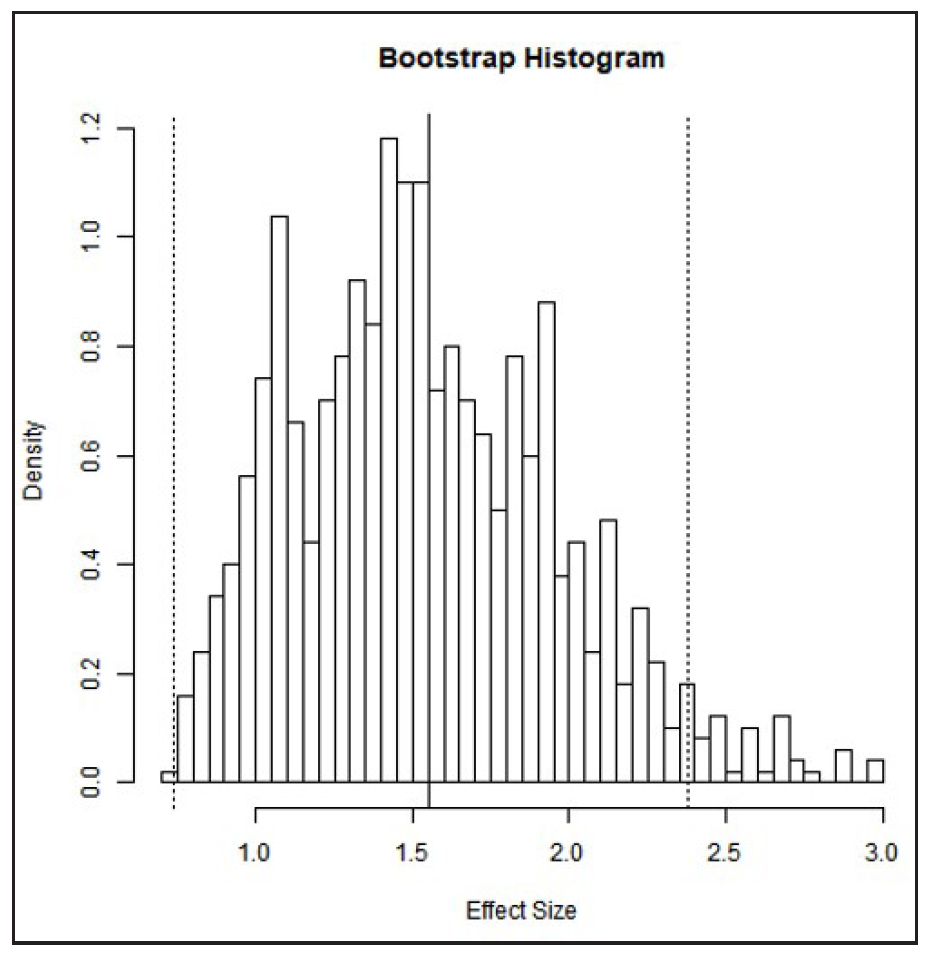
- The histogram of bootstrap analysis using 1000 replicates.
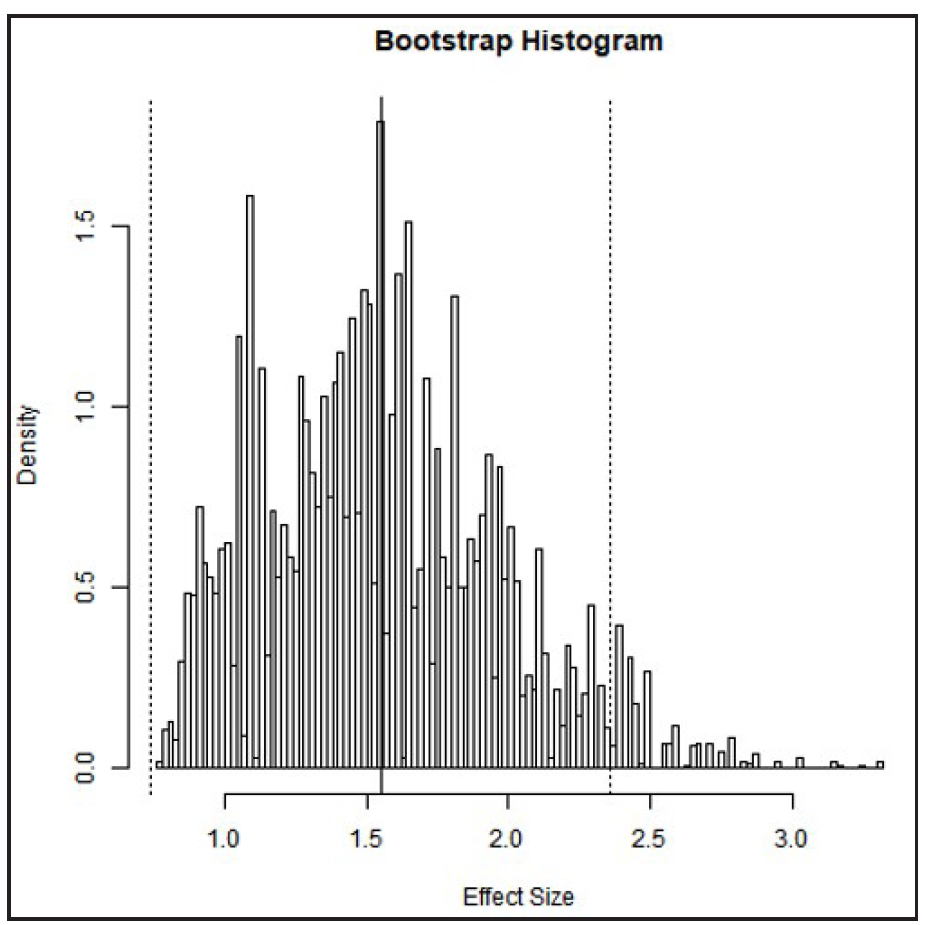
- The histogram of bootstrap analysis using 10,000 replicates.
| Description | Observed effect size (SMD) | 95% Cl | Figure |
|---|---|---|---|
| Meta-analysis (9 studies) | 1.55 | 0.67:2.42 | Fig. 2 |
| Bootstrapped meta-analysis | Mean over the replicates | 95% Cl | |
| Bootstrap replicates: 1000 | 1.54 | 0.74:2.38 | Fig. 5A |
| Bootstrap replicates: 10000 | 1.55 | 0.74:2.36 | Fig. 5B |
CI: Confidence interval, SMD: Standardized mean differences.
DISCUSSION
This meta-analysis reveals that chemerin levels are significantly higher in severe preeclampsia (PE) patients compared to those with mild PE (p < 0.0001). This finding underscores the association between elevated circulating chemerin levels and PE severity. However, substantial heterogeneity (91%) was observed [Figure 2]. Sensitivity analysis identified the study by Murad et al. (2020) as a potential source of this heterogeneity.13 Excluding this study reduced heterogeneity to 59%, while the significant association between higher chemerin levels and severe PE persisted (SMD = 1.13, 95% CI: 0.72 to 1.54, p < 0.0001). This indicates that although the study by Murad et al.13 (2020) contributes to heterogeneity, it does not alter the overall conclusion of increased chemerin levels in severe PE.
The heterogeneity could stem from the younger maternal age and gestational stage (second or third trimester) in the Murad et al.13 (2020) study. Elevated circulating chemerin in severe PE is likely due to increased proinflammatory adipokine synthesis from the placenta during pregnancy.16,17 While the precise mechanisms linking chemerin to PE are not fully understood, recent studies suggest that elevated placental chemerin disrupts normal placental development, contributing to PE onset.18 Chemerin inhibits trophoblast migration, invasion, and tube formation, affecting trophoblast lipid metabolism and promoting pyroptosis and inflammation, thereby playing a role in PE development.19-21
PE is associated with altered body mass index (BMI), insulin resistance, and glucose and lipid metabolic abnormalities, which may contribute to heterogeneity. However, the limited number of studies precluded a meta-regression analysis of these covariates. Nonetheless, recent evidence indicates that chemerin’s association with PE is independent of BMI and obesity status.4,5 The included studies in this meta-analysis reported comparable maternal age, gestational age, and BMI for PE patients. Sensitivity analysis [Figure 4] confirmed the robustness of the finding of elevated chemerin in severe PE, with no single study overly influencing the outcome. Additionally, no significant publication bias was detected [Figure 3]. Despite the limited number of studies, further research on chemerin’s role as a marker for PE detection and severity progression is needed. The accuracy of our meta-analysis was validated through bootstrapped meta-analyses with 1000 and 10,000 replicates, confirming the association of higher chemerin levels with severe PE [Table 2].
Strengths and limitations of this meta-analysis should be noted. Limitations are inherent to the observational study designs included, and heterogeneity may arise from methodological and clinical variations across studies in design, setting, participant selection, and demographic, hormonal, and metabolic parameters. We addressed this through random effects meta-analysis and sensitivity analyses. The quality assessment of individual studies indicated medium to high quality. Due to the limited number of studies available for comparison (only six), it was not feasible to conduct a separate subgroup analysis. Nevertheless, the overall meta-analysis results were robust and stable, as confirmed by sensitivity and bootstrapped analyses. Consequently, this meta-analysis supports the association of higher circulating chemerin levels with PE severity. Further well-controlled studies with larger sample sizes are warranted to evaluate chemerin’s utility for PE diagnosis and disease progression monitoring.
CONCLUSION
This is the first bootstrapping meta-analysis to assess the association of circulating chemerin with PE severity. The results indicate significantly higher serum chemerin levels in severe PE patients compared to mild PE patients, supporting chemerin measurement as a potential method for identifying severe PE. The findings’ validity, robustness, and accuracy are confirmed through sensitivity and bootstrapped meta-analyses. However, further research is needed to explore the diagnostic accuracy of serum chemerin for PE diagnosis and monitoring severity progression.
Authors’ contributions
SRV, MM, SS: Conceptualization; SRV, AA, KG, JJ, AR, AK, BK: Data curation, analysis, methodology and software; MT, SS: Supervision, validation; SRV, MM, AA, KG, JJ: Writing-original draft; BK, MT, SS: Writing-review and editing. All authors approved the final manuscript.
Acknowledgments
SRV personally acknowledges his daughters ‘the Bhairavi Sisters’ (Sahasra and Aagneya) for the time he could not give them during this work, as majority of it was done before 8 AM and after 6 PM (work days), and during non-working Sundays. All the authors thank AK and BK (Department of Biochemistry, AIIMS-Patna) for their assistance in completing this work.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Prevalence of preeclampsia and the associated risk factors among pregnant women in Bangladesh. Sci Rep. 2021;11:21339.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Preeclampsia incidence and its maternal and neonatal outcomes with associated risk factors. Cureus. 2022;14:e31143.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Preeclampsia pathophysiology and adverse outcomes during pregnancy and postpartum. Front Med (Lausanne). 2023;10:1144170.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Circulating chemerin levels in preeclampsia: A systematic review and meta-analysis. Lipids Health Dis. 2023;22:179.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Raised levels of chemerin in women with preeclampsia: A meta-analysis. Biomol Biomed. 2024;24:454-464.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Practice bulletin #33: diagnosis and management of preeclampsia and eclampsia. Obstetrics & Gynecology. 2002;99:159-67.
- [PubMed] [Google Scholar]
- Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Higgins JPT TJCJCMLTPMWV. Cochrane Handbook for Systematic Reviews of Intervention. Cochrane2023 [accessed 2024 Apr 30]. Available from: https://training.cochrane.org/handbook
- GA Wells BSDOJPVWMLPT. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute 2021. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Last accessed 30 Apr 2024].
- Evaluation of Serum Levels of the Adipokines Chemerin and Resistin in Preeclampsia. Life Sci J. 2012;9:1097-8135.
- [Google Scholar]
- Chemerin level in pregnancies complicated by preeclampsia and its relation with disease severity and neonatal outcomes. J Obstet Gynaecol. 2017;37:195-199.
- [CrossRef] [PubMed] [Google Scholar]
- Serum levels of the adipokine chemerin in preeclampsia. J Perinat Med. 2012;40:121-7.
- [CrossRef] [Google Scholar]
- Chemerin Level as a Marker in Preeclampsia and its Relation to the Disease Severity and Neonatal Outcome. Indian Journal of Forensic Medicine & Toxicology. 2020;14:478-84.
- [CrossRef] [PubMed] [Google Scholar]
- Chemerin plays a protective role by regulating human umbilical vein endothelial cell-induced nitric oxide signaling in preeclampsia. Endocrine. 2015;48:299-308.
- [CrossRef] [PubMed] [Google Scholar]
- The predictive value of the first-trimester maternal serum chemerin level for pre-eclampsia. Peptides. 2014;62:150-4.
- [CrossRef] [PubMed] [Google Scholar]
- Serum chemerin levels during normal human pregnancy. Peptides. 2013;42:138-43.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of chemerin and its receptors in the porcine hypothalamus and plasma chemerin levels during the oestrous cycle and early pregnancy. Int J Mol Sci. 2019;20:3887.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Placental trophoblast-specific overexpression of chemerin induces preeclampsia-like symptoms. Clin Sci (Lond). 2022;136:257-272.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- HOXA9-induced chemerin signals through CMKLR1/AMPK/TXNIP/NLRP3 pathway to induce pyroptosis of trophoblasts and aggravate preeclampsia. Exp Cell Res. 2021;408:112802.
- [CrossRef] [PubMed] [Google Scholar]
- Adipokine chemerin overexpression in trophoblasts leads to dyslipidemia in pregnant mice: Implications for preeclampsia. Lipids Health Dis. 2023;22:12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chemerin promotes the pathogenesis of preeclampsia by activating CMKLR1/p-Akt/CEBPɑ axis and inducing M1 macrophage polarization. Cell Biol Toxicol. 2022;38:611-628.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






