Translate this page into:
NAMS task force report on breast cancer in India
Corresponding author: Dr. Sudeep Gupta, Professor, Department of Medical Oncology, Director, Tata Memorial Centre, Homi Bhabha National Institute, Mumbai, Maharashtra, India. sudeepgupta04@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Gupta S, Batra A, Prinja S, Malhotra H, Mohanapriya T, Thomas S, et al. NAMS task force report on breast cancer in India. Ann Natl Acad Med Sci (India). 2025;61:132-70. doi: 10.25259/ANAMS_TFR_14_2024
EXECUTIVE SUMMARY
The National Academy of Medical Sciences (NAMS) has acknowledged the rising prevalence of breast cancer in India as a significant public health concern. In response, NAMS established a task force to prepare this report addressing this critical issue. Given that breast cancer is the most common malignancy among women and a leading cause of cancer-related deaths, there is an urgent need for a comprehensive approach to effectively manage the disease.
The task force conducted a comprehensive review of various aspects of breast cancer diagnosis and treatment across India. Highlighting the alarming rate of nearly 200,000 new cases each year,the group identified significant deficiencies in the healthcare system’s ability to diagnose and treat breast cancer effectively in the country.
One of the most critical gaps identified was in diagnostic services. The availability of radiodiagnostic techniques and pathology services is concentrated in urban centers, resulting in delayed diagnosis in rural areas. The lack of modern imaging equipment and specialized pathology services in many parts of the country means that many patients do not receive the prompt and accurate diagnosis needed for effective treatment planning.
The task force observed a significant gap in the availability of treatment modalities, such as surgery, radiotherapy, and systemic treatments. While urban hospitals often have access to advanced treatment options, semi-urban and rural areas lack basic facilities and trained oncologists, severely hindering the provision of standard care. This disparity is further exacerbated by a general lack of awareness about breast cancer and its symptoms within the community, resulting in a higher proportion of patients presenting with advanced disease stages at the time of diagnosis.
To address these issues, the task force offers several recommendations. First, there is a critical need to enhance the infrastructure for diagnostics and treatment throughout India. This includes increasing the number of diagnostic mammography units and pathology laboratories equipped to diagnose cancer, as well as expanding training programs in oncology subspecialties.
Additionally, the task force emphasizes the importance of implementing nationwide screening programs focused on early detection. By increasing public awareness through comprehensive education campaigns and enhancing the accessibility of screening, the likelihood of detecting breast cancer at an earlier, more treatable stage could be significantly improved.
Furthermore, the task force advocates for a more robust integration of breast cancer care services across all levels of the healthcare system, from primary care to specialized oncology centers. This approach will ensure a continuum of care that is crucial for effective cancer management in the country. We recommend that policymakers prioritize breast cancer as a significant public health issue and support initiatives that facilitate research and development in oncology, improve healthcare access, and ensure the sustainability of cancer care programs.
The comprehensive roadmap provided by the task force outlines a coordinated effort among government agencies, healthcare providers, and community organizations to address breast cancer more effectively. With targeted policies and collaborative strategies, there is potential to significantly improve the outcomes for patients burdened by breast cancer in India through enhanced diagnostics, greater treatment accessibility, and effective public health strategies.
INTRODUCTION
According to the Global Cancer Observatory (GLOBOCAN) 2020 report, 19.3 million new cancer cases were diagnosed globally in 2020, and about 10.0 million deaths were estimated due to cancer.1 Breast cancer emerged as the most common malignancy worldwide, surpassing lung cancer with approximately 2.3 million new cases annually, accounting for 11.7% of all cancer cases1. In India, about 200,000 new cases of breast cancer were reported in 2020, making it the most prevalent cancer among women in and representing nearly 13.5% of the country’s cancer burden.2 The National Cancer Registry 2020 indicates a steady and significant increase in age-adjusted incidence rates (AAR) of breast cancer across many population-based registries, and this trend is expected to continue.2
The diagnosis and treatment of breast cancer involve multidisciplinary teamwork, including specialists in radiodiagnosis, pathology, surgical, medical, and radiation oncology, nurses, counselors, physiotherapists, occupational therapists, and palliative care specialists. Patients suspected of having breast cancer typically present with a breast lump that is evaluated clinically. This is followed by mammography and/or ultrasound, and if findings are suspicious, a core needle biopsy is performed for pathological evaluation. Once a diagnosis is confirmed, most patients require a combination of treatments, including surgery, systemic therapies (chemotherapy, targeted therapy, immunotherapy, hormonal treatment), and radiotherapy. The choice and sequence of treatment modalities depend on the stage of breast cancer (I-IV), the pathological subtype (expression of estrogen receptor [ER], progesterone receptor [PR], and human epidermal growth factor receptor-2 [HER2]), and the patient’s overall fitness to undergo these treatments. Based on the expression of these three markers, breast cancer is classified as hormone receptor (ER and/or PR) positive, HER2 positive, and triple-negative (ER, PR, and HER2).
Breast cancer is treatable and highly curable when diagnosed in its early stages. Significant advances in systemic treatment have improved survival rates for patients with metastatic breast cancer (stage IV); however, it remains an incurable disease. The 5-year overall survival rate are 95% for stage I, 92% for stage II, 70% for stage III, and only 21% for stage IV patients. In India, the survival rates for patients with breast cancer are lower compared to Western countries due to several factors, including the late stage presentation, delayed initiation of definitive management, and inadequate or fragmented treatment.
Therefore, there is an unmet need to improve outcomes for patients with breast cancer in India. This requires an integrated, multipronged approach that encompasses early diagnosis, timely referral, and affordable access to standardized multimodal treatment. This white paper, developed under the auspices of the National Academy of Medical Sciences, outlines several strategies to address these challenges.
BACKGROUND
Breast cancer poses a significant public health challenge in India, with its prevalence steadily rising over the years. Recognizing the urgent need for comprehensive strategies to address this issue, the National Academy of Medical Sciences (NAMS) has undertaken a proactive initiative. In alignment with its commitment to promoting health equity and advancing medical interventions, NAMS has constituted a task force to develop guidelines for stakeholders involved in combating breast cancer across the Indian population. The NAMS has establishment a task force on breast cancer which comprises experts from various medical fields, research institutions, and public health agencies. This task force is charged with the crucial mandate of drafting a white paper intended to serve as a comprehensive document that provides insights and recommendations for policymakers to enhance intervention activities related to breast cancer in India.
TERMS OF REFERENCE (TORS) FOR THE TASK FORCE
The main objectives of this task force include the following:
-
1.
To identify the current status of breast cancer in the country.
-
2.
Identify the deficiencies which need to be addressed.
-
3.
To recommend measures for improving the interventions in the area of breast cancer.
METHODOLOGY
An initial online meeting of expert members was held on 08/02/2024, where the objectives and broad framework of the white paper were discussed and agreed upon by the members of the task force. The task force conducted a thorough examination of data and peer-reviewed publications relevant to their specialties for breast cancer. Through this process, consensus was reached on significant observations and recommendations, taking into account the diverse healthcare services and socio-cultural and economic contexts prevalent throughout the nation. The initial draft was compiled from inputs provided by several experts involved in the management of breast cancer, and consensus was achieved by circulating the draft among task force members for review and feedback. Subsequent modifications were made based on the suggestions received, and all members have approved the final version of the draft. This iterative process ensured that the document was comprehensive and reflective of the collective expertise and perspectives of the task force members.
OBSERVATIONS/CRITICAL REVIEW
Current situation in the country
Epidemiology
According to the GLOBOCAN 2020, 19.3 million new cancer cases were diagnosed globally in 2020, and about 10.0 million died.1 These cancer cases (excluding non- melanomatous skin cancers) are estimated to rise to 26 million in 2040, according to the report. Current cancer incidence rates are three times higher in high-income countries (HIC) as compared to low- and middle-income countries (LMIC).3 However, LMICs are expected to be largely responsible for the increase in cancer incidence worldwide over the next 50 years.4 Sixty percent of the global population resides in Asia, which accounts for 50% of cancer cases and 58% of cancer-related deaths. Furthermore, India ranks third in accounting for cancer cases, following China and the United States of America.5 The projected cancer burden in India is estimated to reach 2.08 million by 2040, representing a 57.5 % increase from 2020.
Breast cancer is the most common malignancy among women globally and in India. Approximately 200,000 new cases were reported in 2020, and it is expected that 232,832 cases will be diagnosed in 2025.2 A higher incidence of breast cancer has been reported in urban population-based cancer registries (Hyderabad, Delhi, etc.) compared to rural registries (Barshi, Osmanabad, etc.). Among the data reported by hospital-based cancer registries, most diagnosed cases of breast cancer in females showed locoregional spread (57.0%), followed by localized disease (29.0%) and distant metastasis (10.3%).2 A recent study from Mumbai (Tata Memorial Centre ) and Pune analyzed 912 patients diagnosed with breast cancer. The median age at diagnosis was 47 years (range, 23 to 85 years); 63.2% had pathologically confirmed axillary lymph node involvement, and 54% of patients had ER-positive disease. Approximately one-quarter of patients had HER2 - positive disease and, triple-negative breast cancer (TNBC) was present in 266 (29.1%) patients.6
Diagnostics: Radiodiagnosis and pathology
Radiodiagnosis imaging has two main categories of indications: Screening the healthy population for early detection of breast cancer and diagnostic breast imaging. The latter involves evaluating of women with breast complaints to determine whether the findings are cancerous or non-cancerous. Imaging is also required for clinical staging, treatment planning for breast cancer, and monitoring individuals who have been treated for breast cancer.
Mammography: Mammography is the primary imaging modality for breast cancer. Other modalities, such as ultrasound, MRI, and positron emission tomography - computed tomography (PET-CT), serve as supplementary tools to mammography in specific contexts. Mammography screening has been extensively studied and is a time-tested method proven to reduce breast cancer-related deaths in certain age groups. However, it is a resource-intensive approach, predominantly available in a few developed countries worldwide.
Currently, there is no structured population-based mammography screening program in the country and it is unlikely to be feasible in the foreseeable future due to its resource-intensive nature and the large population. For example, the number of mammography machines in India is only about 5% of that in the USA, despite India having a population more than three times that of the USA. A negligible proportion of women population in India undergo self-volunteered and self-funded periodic mammography examinations as a part of regular health check- ups in private hospitals for early detection of breast cancer, known as opportunistic screening. However, the protocol and standard of these screening are highly variable, and no precise data on the number of examinations or their benefits are available. A few small-scale, sporadic mammography screening studies have been conducted in India, sometimes utilizing mobile machines. Some of these are single-round assessments that show prevalence rather than incidence of breast cancer, and others lack long-term follow-up or mortality data.
As population-based mammography screening is not feasible in our country, promoting awareness about breast cancer and its early symptoms is crucial. The benefits of seeking immediate medical attention and the importance of early-stage diagnosis should be emphasized. While self-breast examination and clinical breast examination have been studied in other countries, they have not proven effective in reducing breast cancer-specific mortality. However, a long-term population screening study conducted by Tata Memorial Hospital (TMH), which utilized clinical breast examination by trained healthcare workers, demonstrated that this approach can help downstage the disease at presentation and may reduce mortality to some extent in specific age groups.7 This approach can be further explored on a large scale.
In India, breast imaging is primarily employed to evaluate women presenting with breast-related complaints, such as pain, lumps, and/or discharge, which is referred to as diagnostic breast imaging. This approach is useful for diagnosing breast diseases, cancer, or non-cancer. For women suspected of having breast cancer, imaging aids in confirming or ruling out breast cancer. If imaging indicates the presence of cancer, it is also used to obtain an image-guided biopsy for pathological confirmation. Once breast cancer is confirmed, imaging is used for staging, deciding on management, accessing the response to non-surgical management, and detecting recurrence in treated patients.
In pathology services, the following observations have been made:
Routine histopathology of breast cancer: The primary issue with pathology reporting of breast cancer in India is the lack of reporting essential features in histopathology reports and improper documentation of biomarkers. In a study conducted by the National Cancer Grid External Quality Assurance Scheme (NCGEQAS) at Tata Memorial Hospital in 2019, slides from a breast cancer excision were circulated for minimum data set reporting (unpublished observations). Overall, 89 out of 94 centers (95%) provided a concordant diagnosis, while five centers (05%) rendered a discordant diagnosis of invasive lobular carcinoma. The most significant challenges were observed in reporting lymph node status and margin assessments.
Biomarker reporting: The reported positivity rates for hormone receptors in breast cancer from India vary from 32% to 70%, indicating heterogeneity in testing practices across the country.8 According to American Society of Clinical Oncology-College of American Pathologists (ASCO-CAP) recommendations, key reasons for the underestimation of hormone receptors include delayed transport of excised specimens, delayed fixation, and insufficient monitoring of cold ischemia time. Pathologists often hesitate to document delays in specimen transport due to concerns about potential of persecution by patients or surgeons. While poor fixation is the main issue for ER/PR testing, HER2 results are affected by the use of non- in vitro diagnostic (IVD) or non- Food and Drug Administration (FDA)-approved antibodies and the lack of automated platforms nationwide. The reported positivity for HER2 in breast cancer ranges from 16% to 30%.9,10 In the most recent NCGEQAS run in 2024 for HER2 immunohistochemistry, only 63 out of 172 participating centers (36.6%) centers used the FDA-approved Ventana 4B5 antibody on the Ventana machine, indicating significant heterogeneity in the use of the FDA-approved tests in laboratories. (Unpublished observations). The NCGEQAS experience has recently been published highlighting the increasing use of FDA-approved HER2 testing in consistently participating centers, leading to improved laboratory performances.11 Interpretative errors in the HER2 test are common in centers lacking molecular diagnostic facilities, resulting in pressure on these services to perform more HER2 tests than usual. For the programmed death-ligand 1 (PDL1) assay in triple-negative cancer, pembrozulimab is the recommended drug, with the companion test being the PDL22C3 antibody performed on a Dako link48 machine. Most laboratories in India have Ventana machine installed due to the availability of numerous FDA approved antibodies, making the use of PDL22C3 antibody on a Dako machine impractical. In a NCGEQAS exercise, only 25 out of 118 laboratories that perform immunohistochemistry volunteered for this cycle. All laboratories except one used the VENTANA SP263 for PDL1 testing, indicating a lack of capability to perform the other test. Only one laboratory utilized the Dako 22C3 for this purpose. Despite this, overall test performance was commendable, with only 3 out of 19 centers reporting discordant results. While this is encouraging, it suggests that most centers in India are not equipped to conduct PDL1.
Routine molecular testing in breast cancer for biomarkers: The primary biomarker regularly tested in breast cancer is HER2, using fluorescent in situ hybridization (FISH). However, this testing requires the establishment of molecular pathology services within Institutes and the presence of trained personnel. As HER2 testing has become mandatory, complex profiles are emerging. However, the practice of separating FISH from surgical pathology by sending samples to reference laboratories carries a risk of errors. Options and alternatives for FISH are discussed below.
Advanced molecular diagnostic and surrogates: In addition to ER, PR, and HER2, several other tests guide therapy decisions in breast cancer, including PI3k and ESR1 mutations in hormone-positive cancers, homologous recombination deficiency (HRD) in triple-negative breast cancers, and oncotype Dx for luminal cancers. Biomarkers that predict the need for chemotherapy in hormone-positive early breast cancer are essential; clinicians typically consider factors such as age, nodal status, Ki67, and gene expression profiling, with OncotypeDx being the standard choice recommended by most international guidelines. However, for economic reasons, many clinicians in India rely on alternate tests like Ki67 and CanAssist. There is insufficient evidence to confirm that high Ki-67 levels predict the efficacy of adjuvant chemotherapy or that patients with Ki-67-low breast cancer do not benefit from it.12 Challenges associated with Ki67 include varying cut-off values, different counting methods, and significant effects from delayed fixation.13 A study at our institute found a significant difference in Ki67 values between breast cancer samples fixed immediately and those fixed after 1 to 6 hours. Lin’s concordance correlation coefficient (0.5350) indicated poor agreement between the two fixation times, highlighting the need for oncologists to be aware of how delayed fixation impacts Ki67 results. In a Delphi survey of oncologists in India, 84% preferred CanAssist, while 80% favored OncotypeDx for risk stratification.14 CanAssist utilizes five immunohistochemistry biomarkers—CD44 (a stemness marker), N-Cadherin and pan-Cadherin (cell adhesion and invasion markers), and ABCC4 and ABCC11 (drug exporters)—which are not proliferation markers but have shown some ability to predict better outcomes compared to traditional factors.15 While these tests provide better valuable information, it is essential to critically evaluate their scientific basis and the data behind them as these are often commercially driven. Looking towards IHC-4 translation and other gene-based options would be a more robust choice in India. HRD testing is available through several commercial platforms, including the gold standard Myriad myChoice CDx test for patients who can afford. However, HRD testing requires a NextSeq or HiSeq platform which is not feasible in many laboratories at present. Limited panel testing using MiSeq is the preferred method for Pi3K and BRCA mutation testing as an alternative to HRD tests in Indian patients. Most molecular tests are outsourced to commercial laboratories due to lack of resources and financial backing in institution-based laboratories. Consequently, effective marketing strategies from commercial laboratories have hindered the development of molecular diagnostics in even large cancer centers.
Treatment: Surgery, systemic treatment and radiotherapy
Surgery: The proportion of patients diagnosed with breast cancer at a younger age is notably higher in India compared to the high-income countries. In some studies, the median age of breast cancer presentation in India was found to be just 45 years; this is in sharp contrast to the USA, where the median age of presentation was 61 years.16 Additionally, patients in India tend to present with later stage disease compared to the West. More than 60% of patients in India present at Stage 3 or 4, while around 60% of patients in the USA are diagnosed at in-situ or Stage 1.
Delay in seeking healthcare: More than 50% of patients in India experience a delay of over 3 months before seeking medical care.
Delayed definitive management/inadequate treatment: Regarding 5-year overall survival rates, studies report 95% for stage I, 92% for stage II, 70% for stage III, and only 21% for stage IV patients.10,16 The survival rate for breast cancer patients in India is lower compared to Western countries due to factors such as earlier age at onset, late stage presentation, delayed initiation of definitive management, and inadequate or fragmented treatment.17,18
Limited Research Output: Despite the high burden of breast cancer in India, there is a lack of indigenous research contributing to global knowledge and innovation in breast cancer treatment. The high prevalence of poor-prognosis early-age breast cancer, despite the presence of low-risk hormonal profiles (early age at first childbirth, multiple pregnancies, prolonged breastfeeding, low prevalence of nulliparity, limited use of hormone replacement therapy, etc.), suggests the likely influence of as yet undetermined genetic, dietary, or environmental factors. However, due to the enormous patient load, specialists often lack time to dedicate to research.
Systemic therapy: Systemic therapy is a crucial component of modern breast cancer treatment, delivered by medical oncologists. It includes a range of drugs, such as chemotherapy, endocrine therapy, HER2 targeted therapy, other targeted therapies, and immunotherapy (primarily immune checkpoint inhibitors). Many generic, affordable versions of these drugs are available and are included in central and state health schemes in India. However, targeted therapies and immunotherapy are often expensive and not easily accessible due to financial constraints faced by the majority of breast cancer patients in the country. The following sections address some of the issues and potential solutions to enhance access to essential breast cancer drugs, as well as the infrastructure and expertise needed to administer them to eligible patients.
Radiotherapy: Radiotherapy of the remaining breast post-surgery is an integral part of the breast cancer treatment, delivered by radiation oncologists. It can result in up to 50% improvement in local control, which translates to saving approximately one life for every four local controls achieved.19 In patients with node-positive cancer, post- mastectomy radiotherapy leads to a reduction of up to 69% in the relative risk of loco-regional recurrence, preventing one death for every 1.5 loco-regional recurrences avoided over 20 years.19,20 The effectiveness of this treatment has been established across various tumor sizes and grades, regardless of the nodal burden. Additionally, regional nodal radiotherapy has been found to offer further benefits for patients with more advanced cancers. Notably, studies from India demonstrate comparable benefits of radiotherapy to those seen in stage-matched patients from Western data, confirming its effectiveness amongst Indian women.21,22
Hospital-Based Cancer Registry (HBCR) data revealed that 57% of patients presented with loco-regional disease, while 29% present with localized cancer.2 Consequently, it is expected that over 85% of breast cancer patients will require radiotherapy during their lifetime, with the majority being potentially curable. However, many patients in India face a significant burden of locally advanced, incurable breast cancers requiring optimal symptomatic control.16 For these patients, radiotherapy offers effective and durable symptom management, helping to maintain their quality of life while also improving objective response rates to the treated breast tumor.23
Current infrastructure, facilities, technologies, policies, programs, etc., in the country in the context of the problem/health issue
Human resource and healthcare system
The landscape of cancer care in India has seen significant advancements in treatment modalities and preventive measures. Human resources are crucial at various levels of healthcare delivery, as illustrated in Figure 1.
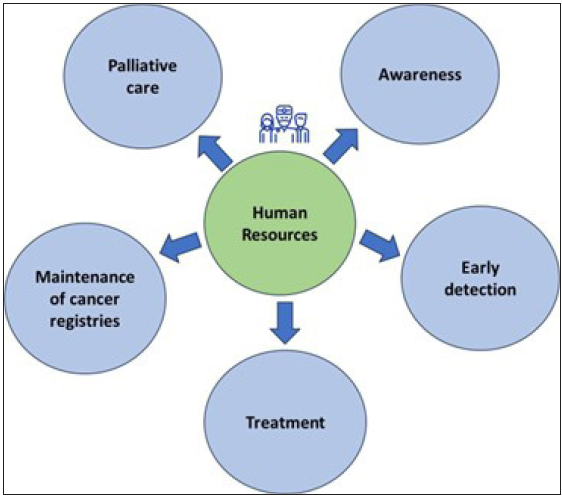
- Human resources for cancer care.
Through a multifaceted approach that includes awareness campaigns, wellness promotion, and targeted screenings- especially for women over 30 years of age at the community level, the initiative aims to reduce the cancer burden of cancer by identifying cases at earlier stages when treatment outcomes are more favorable.24 Accredited Social Health Activists (ASHAs) play a pivotal role in this effort by gathering crucial information on breast cancer risk factors using community-based assessment checklists. This grassroots strategy helps identify individuals at higher risk, enabling timely interventions and referrals for further evaluation. At primary healthcare units, a diverse group of healthcare professionals, including community health officers, auxiliary nurse midwives, medical officers, and staff nurses, collaborate to conduct clinical breast examinations. Suspected cases are promptly referred for comprehensive evaluation, ensuring no potential malignancies are overlooked. Additionally, these frontline healthcare providers offer vital follow-up and palliative care services throughout patients’ treatment journey, emphasizing holistic support and patient-centered care.24
The extensive reach of the Ayushman Arogya Mandir initiative is noteworthy, with over 1,63,402 operational centers by the end of 2023. Within these centers, primary healthcare teams have conducted a staggering 10.04 crore breast cancer screenings, underscoring the program’s commitment to widespread outreach and accessibility.25 Furthermore, the integration of yoga sessions highlights a holistic approach to wellness promotion and supportive care, addressing both physical and psychosocial needs.
In parallel, the establishment of non-communicable disease (NCD) clinics at community health centers (CHCs) and district hospitals under the national program for control of non-communicable diseases further strengthens the continuum of care. Nurses and doctors stationed at these facilities play a crucial role in facilitating early diagnosis, managing complicated cases, and coordinating follow-up chemotherapy at the district level.26 The treatment of breast cancer is concentrated at tertiary-level health facilities and specialized cancer care centers staffed with oncologists. The staff provides comprehensive cancer care, conducts training of health personnel, maintains cancer registries, and generates evidence through rigorous research activities. However, despite these significant strides, challenges persist in ensuring equitable access to breast cancer screening and treatment services across India.
A growing proportion of cancer cases is directly related to the increased demand for cancer care services. For instance, the number of patients estimated to need first- course chemotherapy in LMICs is projected to rise from 6·2 million to 10·0 million annually between 2018 and 2040, accounting for approximately 63%-67% of the global estimated patients requiring first-course chemotherapy27 [Figures 2 and 3].
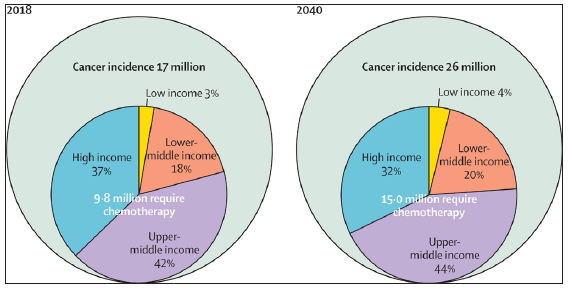
- Growth in cancer incidence and chemotherapy demand between 2018 and 2040 stratified by income level. Source: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(19)30163-9/abstract
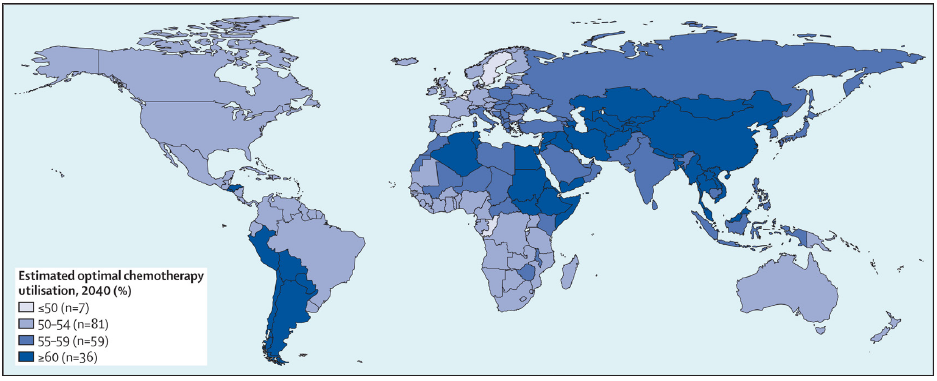
- Estimated optimal chemotherapy in 2040 by country. Source: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(19)30163-9/abstract
Similarly, the projected number of patients diagnosed with cancer requiring radiotherapy globally is expected to reach 12 million by 2035.28 The estimated number of required radiotherapy fractions is projected to exceed 204 million worldwide by that same year, with India accounting for 18 million of those required fractions.
The proportion of cancer patients receiving treatment for different types of cancer varies significantly worldwide and is lower in many LMICs.27 These disparities may arise from issues related to access and availability of cancer care services. Inadequate service delivery results from a confluence of factors, including lack of resources for timely and accurate cancer diagnosis, poor infrastructure to support cancer care, a shortage of trained health personnel, and restricted access to medications due to high costs and supply-chain issues.27 In India, cancer care is primarily concentrated in tertiary hospitals and major cancer centers in urban areas. However, among other factors, inadequate infrastructure and a lack of human resources make it challenging to provide high-quality cancer care.29
Existing health system framework for the provision of cancer care in India
To address the threat of cancer in India, the Government of India has established 599 NCD Clinics at the district level and 3,274 NCD Clinics at the community health center level under the NPCDCS. Additionally, the flagship national insurance program, Ayushman Bharat Pradhan Mantri Jan Aarogya Yojana (ABPM- JAY), was launched.
In 2018, India embarked on an ambitious path to enhance its health system and provide quality cancer care. At the population level, initiatives under the National Health Mission (NHM) were rolled out in over 215 districts to prevent, control, and screen for common NCDs (diabetes, hypertension, and common malignancies such as oral, breast, and cervical cancers). Screening for oral, breast, and cervical cancers is a critical component of service delivery under Ayushman Arogya Mandirs (formerly known as Health and Wellness Centers) implemented at the primary health care level [Figure 4]. Other initiatives, such as promoting healthy eating and regular physical activity, aim to encourage healthier lifestyles. The central government is also implementing the strengthening tertiary care for cancer scheme to improve cancer care facilities at the tertiary level. By 202, six State Cancer Institutes were operational, and the establishment of 19 additional State Cancer Institutes (SCIs) and 20 Tertiary Care Cancer Centers (TCCCs) has been approved.30 Furthermore, oncology is a focus area for the new AIIMS facilities and many upgraded institutions under the Pradhan Mantri Swasthya Suraksha Yojana (PMSSY). In Indian cities like Bhopal, Patna, Bhubaneswar, Jodhpur, Raipur and Rishikesh the new All India Institute of Medical Sciences (AIIMS) are fully functional.30
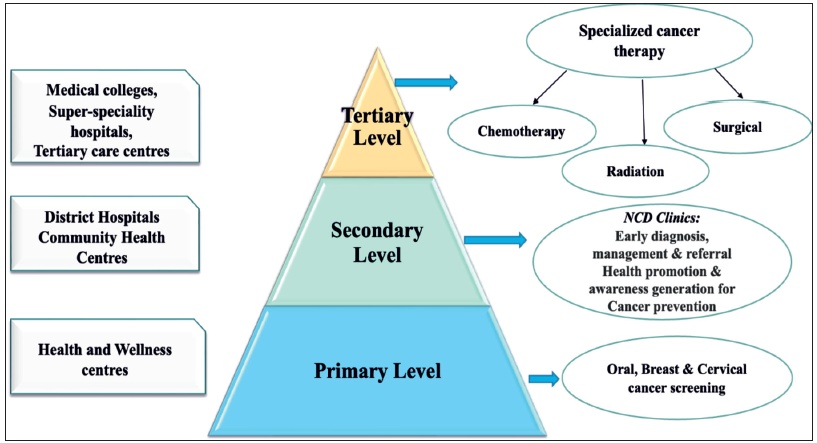
- Indian health system framework – cancer care. NCD: Non-communicable disease
Diagnostics: Radiodiagnosis and pathology
Radiodiagnosis: This includes both machines and manpower. Currently, there are approximately 3000 mammography units in India with almost all located in metro or tier 1 cities, primarily in private hospitals, diagnostic centers, or labs. Given that there are over 200,000 new cases of breast cancer each year in India, the current availability and accessibility of mammography facilities are highly inadequate for timely diagnosis and treatment. Additionally, significant variations in the quality, efficacy, and reliability of mammography machines further complicate the situation. Mammography is highly sensitive to quality control, and is the most regulated and legislated imaging modality in developed countries. In India, AERB oversees the radiation safety of these machines. However, there is no central regulatory authority or legislation to ensure quality assurance and clinical standards. Moreover, less than 10% of these machines are state-of- the-art full-field digital mammography machines that meet contemporary international standards, while the remainder are mostly suboptimal or outdated models.
Ultrasound: Ultrasound is the second most used breast imaging modality typically serving as a supplement to mammography, especially in younger women who often have mammographically dense breast tissue. In such cases, mammography is less sensitive for detecting breast cancer. However, breast ultrasound can be an effective primary modality of breast imaging in women under 35 years of age. It is also useful for diagnosing breast diseases, including cancer, in women presenting with symptoms when mammography is unavailable. Furthermore, ultrasound is the most commonly used modality for obtaining image-guided breast biopsy to establish or rule out the pathological presence of breast cancer. Limitations of ultrasound include higher operator dependence, less reproducibility, and comparatively lower sensitivity in detecting very small cancers. While it has not been extensively studied for population or community screening for breast cancer, ultrasound equipment and the expertise to use it are widely available across India, including in tier 2 and tier 3 cities, as well as in government and private centers, unlike the limited access to mammography. It has also been recognized that the average age of breast cancer onset in India is lower than that in Western countries. Given the established effectiveness of ultrasound in younger women and its widespread availability, it has significant potential to be used for early breast cancer detection. However, large studies are needed to generate data to support this hypothesis.
Breast MRI is the most sensitive breast imaging modality for diagnosing breast cancer, but it is also the most expensive and time-consuming. Consequently, it is used only in specific situations, such as for women with equivocal mammograms and women with a genetically high risk of breast cancer. It can also serve as a problem-solving imaging modality. Given its specialized indications, MRI are very specific and not widespread, and it may not be a priority in the context of public health. However, the necessary equipment and expertise are available in highly specialized tertiary care centers to meet its requirements.
Nuclear medicine techniques, such as radionuclide scans for breast scans or bone scans for the detection and staging breast cancer, have been commonly used in the past. However, with the advent of PET-CT and its growing availability in major cities, it has become the preferred modality as a single test for staging workup. It is also useful to monitor treatment responses in patients with metastatic breast cancer. Occasionally, PET-CT used in cases of small and early-stage cancer, even though most guidelines do not recommend it for those situations. Therefore, judicious use of this modality needs to be encouraged, as it is quite expensive.
Imaging-guided breast biopsy and other interventional procedures are essential components of breast cancer diagnosis and management. In majority of the cases, accurate diagnosis of breast cancer is mostly made on ultrasound-guided biopsy. In specific situations, mammography-guided stereotactic breast biopsy or MRI-guided breast biopsy is utilized. In addition to biopsies, pre-therapy tumor marker clip placement or pre-operative hook-wire localization of non-palpable breast cancers, performed by a team of specially trained radiologists and radiographers, are crucial procedures in modern breast cancer treatment protocols.
With the insufficient infrastructure for modern breast imaging facilities, which are primarily available only in large centers, it is crucial to optimize their use. Both under and over-utilization should be avoided. To achieve this, primary care doctors may be regularly updated on recent developments and best practices for using breast imaging techniques, including nuclear medicine. Ordering mammograms for very young women or too frequently should be avoided. Similarly, the overuse of MRI or PET-CT should be discouraged.
Manpower training for breast imaging: Mammography requires specially trained personnel, which includes radiologists and technologists (radiographers), who are well-versed in mammography. Mammography is included in the curriculum of post- graduate teaching courses, such as Doctor of medicine (MD) or Diplomate of National Board (DNB) in Radio-diagnosis across the country. However, state of the art mammography machines are available only in a few premier government and private medical colleges. Many medical colleges either have outdated or non-functional mammography machines, or they lack them entirely. Notably, some reputed government medical colleges in the national capital do not have functional digital mammography facilities. The situation makes it challenging to train and produce an adequate number of radiographers or radiologists specialized in mammography.
In the last decade, awareness of the need for specialist radiology manpower has increased. Specialty training for breast radiologists and radiographers is offered at a few tertiary care institutions like AIIMS, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, and other medical institutes of national importance, as well as at a few corporate hospitals that provide post-doctoral fellowship courses in breast imaging or women’s imaging. While these institutions produce quality human resources the number generated every year remains highly inadequate. Given the limited expertise in modern breast imaging facilities, which are mostly concentrated in premier hospitals, encouraging short observer ships or basic training for radiologists from peripheral centers at nearby tertiary care centers may be beneficial.
Qualified breast radiologists who have been trained in premier Indian institutes, as well as those from international specialty centers practicing in India, came together to form the Breast Imaging Society of India about ten years ago. This professional society has actively conducted regular conferences and workshops to constantly update the knowledge and skills of breast imaging and interventions for radiologistsand radiographers across the country. These efforts have positively contributed to addressing the challenges in the field.
Pathology: Current infrastructure, facilities, technologies, policies, programs, etc., in the country concerning the health issue reveal that basic pathology services are still primarily concentrated in cancer institutes in India, but cancer surgeries are performed throughout the country, and the sample is transported to a “state-of-the-art” pathology center for diagnostics. However, the damage done by delayed fixation in formalin cannot be undone, and this is the root problem of many failed or discordant biomarkers in the country. Additionally, the FDA-approved antibodies are expensive, prompting laboratories that provide immunohistochemistry-based diagnostics to use non-IVD (in vitro diagnostic) antibodies, which can exhibit batch-to-batch variation. Automation in immunohistochemistry ensures uniformity due to locked protocols; however, these systems are complex and high maintenance and only available in centers with high workloads. Most international antibody companies are promoting the licensing of ready-to-use antibodies due to the issue of dilution impacting test results. This, coupled with licensing norms introduced in the country for two years, has greatly reduced the quality of immunohistochemistry in the country.
Treatment, including surgery, systemic treatment, and radiotherapy
Surgery: Infrastructure and Facilities: India has been expanding its healthcare infrastructure, including cancer treatment facilities, across the country. Many hospitals now feature dedicated breast cancer clinics with state-of-the-art diagnostic and treatment capabilities.
Some notable cancer treatment centers in India include Tata Memorial Center in seven states in India including Mumbai, the AIIMS in New Delhi, and various regional cancer centers throughout the country.
Advanced breast cancer diagnosis and treatment technologies, such as digital mammography, ultrasound, MRI, PET-CT scans, and molecular profiling, are increasingly available in urban areas and major healthcare institutions.
Adopting telemedicine and teleconsultation services has also facilitated access to expert opinions for patients in remote areas.
The Indian government has initiated several policies aimed at improving cancer care, including breast cancer. For instance, the NPCDCS seeks to strengthen infrastructure, human resources, and screening programs for the early detection and management of cancer.
The government has also introduced various health insurance schemes to financially assist cancer patients with treatment expenses.
Various awareness and screening programs for breast cancer have been implemented at both national and regional levels. These programs aim to educate women about breast health, promote regular screenings, and facilitate early detection. NGOs and advocacy groups also play a crucial role in conducting awareness campaigns, providing patient support services, and advocating for policy changes to enhance breast cancer care.
Systemic treatment: Systemic treatment includes chemotherapy, targeted therapy, hormonal therapy, and immunotherapy, forming an integral part of multimodal treatment. The aim of administering systemic treatment is to control micrometastasis in early-stage diseases and prolong the survival of metastatic diseases. The following section discusses the absolute or relative benefit of administering such therapies.
Chemotherapy: In (neo)adjuvant settings, anthracyclines (doxorubicin or epirubicin) and taxanes (paclitaxel or docetaxel) form the backbone of chemotherapy. In the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis, the administration of an anthracycline-based chemotherapy regimen reduced the risk of recurrence from 47% to 39% (relative risk [RR], 0.73; 95% confidence interval [CI], 0.68-0.79), and the risk of breast cancer mortality decreased from 36% to 29% (RR, 0.79; 95% CI, 0.72-0.85) at ten years compared to no adjuvant treatment.31 Furthermore, the addition of a taxane agent reduced the 10-year risk of recurrence from 39% to 36% (RR, 0.87; 95% CI 0.82-0.93) and the risk of breast cancer mortality from 28% to 24% (RR, 0.88; 95% CI, 0.82-0.95).32 The benefits of additional taxane were independent of age, nodal status, tumor size, tumor grade, and estrogen receptor status.
In certain clinical scenarios, a combination of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) is used, providing an advantage similar to that of four cycles of anthracycline-based chemotherapy.31
The addition of carboplatin in the TNBC subtype has been debated over the last few decades. However, a recently reported large randomized controlled trial from TMH, Mumbai, and a meta-analysis support the use of carboplatin in younger patients.33 The benefit was limited to those patients aged ≤ 50 years, who showed an 11.2% increase in overall survival, with rates of 65.9% vs 77.1%, respectively (hazard ratio [HR] =0.611; P=0.003).34 Additionally, the use of capecitabine in patients with stage I-III TNBC who have residual disease after receiving neoadjuvant chemotherapy improved disease-free survival to 69.8% in the capecitabine group compared to 56.1% in the control group (HR, 0.58; 95% CI, 0.39 to 0.87), and the overall survival rate to 78.8% versus 70.3% (HR, 0.52; 95% CI, 0.30 to 0.90).35
Endocrine therapy: Tamoxifen for five years is associated with a decreased risk of breast cancer recurrence (24.8% vs 37.7%; RR, 0.63; 95% CI, 0.58-0.68) and mortality due to breast cancer (23.9% vs 33.1%; RR, 0.70; 95% CI, 0.64-0.75).36 Extending tamoxifen by another five years further reduced the recurrence by approximately 4% and breast cancer mortality by 3%.37 Aromatase inhibitors (anastrozole, letrozole, exemestane): Aromatase inhibitors are preferred over tamoxifen for adjuvant hormonal therapy in postmenopausal women with hormone-receptor-positive breast cancer. In the comparisons of aromatase inhibitors versus tamoxifen, recurrence RRs significantly favored aromatase inhibitors during years 0-1 (RR, 0·64; 95% CI, 0·52-0·78) and 2-4 (RR 0·80, 0·68-0·93), though not significantly thereafter. The 10-year breast cancer mortality was lower with aromatase inhibitors than with tamoxifen (12·1% versus 14·2%; RR, 0·85; 95% CI, 0·75-0·96).38 Furthermore, no significant difference has been demonstrated among the various aromatase inhibitors.39–41
In premenopausal women at higher risk of breast cancer recurrence as assessed by tumor characteristics like nodal involvement, suppression of ovarian function is associated with a reduction in the 15-year risk of recurrence by 12·1% (28·9% vs. 41·0%; RR = 0·70, 0·63–0·78), 15-year breast cancer and all-cause mortality by 8·0% (20·9% vs 28·9%; RR 0·69, 0·60–0·80) and 7.2% (26·0% vs. 33·1%; RR = 0·73, 0·64–0·82), respectively.42 GnRH agonists, including leuprolide and goserelin, are commonly used for ovarian function suppression for a duration of five years.
Bone-modifying agents, including zoledronic acid and denosumab, are often prescribed alongside hormonal treatment to reduce the risk of skeletal events (like fracture) associated with bone metastasis and osteoporosis caused by aromatase inhibitors.43,44
HER2-targeted therapy: Patients with HER2-positive breast cancer are typically treated with neoadjuvant chemotherapy and targeted therapy. Trastuzumab, an anti- HER2 antibody, when added to chemotherapy, has been shown to decrease the risk of recurrence of HER2-positive breast cancer by 9% (HR, 0.66, 95% CI 0.62-0.71) at ten years. The higher the tumor’s risk, the greater the absolute reductions in five-year recurrence (e.g., 5.7% in N0 disease, 6.8% in N1 to N3 disease, and 10.7% in N4+ disease). Breast cancer mortality at ten years is reduced by 6.4% (HR 0.67, 95% CI 0.61-0.73).45 The optimal duration of trastuzumab is 12 months; however, the most benefit is derived from the first six months of treatment, which may be considered essential.46
A newer HER2 antibody, pertuzumab, in addition to trastuzumab and chemotherapy backbone, showed a modest improvement in breast cancer recurrence and overall survival in the overall population. However, a preplanned subgroup analysis in patients with the node-positive disease showed that pertuzumab improved the six-year disease-free survival (88 versus 83 per cent; HR 0.72, 95% CI 0.59-0.87).47
An antibody-drug conjugate, trastuzumab emtansine (TDM-1), when administered in patients with HER2-positive breast cancer who have residual tumors after neoadjuvant systemic treatment, significantly improved disease-free survival (88% versus 77%; HR, 0.50; 95% CI, 0.39-0.64) and overall survival (89% versus 84% at seven years; HR, 0.66; 95% CI, 0.51-0.87).48
In metastatic settings, first-line treatment with trastuzumab and pertuzumab in addition to docetaxel was associated with a significant overall survival benefit compared to trastuzumab and docetaxel (57·1 vs 40·8 months; HR, 0·69; 95% CI, 0·58-0·82). In second line treatment, trastuzumab deruxtecan, demonstrated a significant improvement in progression-free survival compared to trastuzumab emtansine, (28.8 vs. 6.8 months; HR, 0.33; 95% CI, 0.26-0.43).49 However, due to prohibitive cost, trastuzumab emtansine remains the most commonly used second-line agent, which also showed an improvement in overall survival compared to lapatinib and capecitabine (30.9 vs. 25.1 months; HR, 0.68; 95% CI, 0.55 to 0.85).50
Other targeted therapies: Olaparib: In patients with high-risk, HER2-negative early breast cancer and germline BReast CAncer1 (BRCA1) or BReast CAncer2 (BRCA2) pathogenic or likely pathogenic variants, adjuvant olaparib for one year after completing local treatment and neoadjuvant or adjuvant chemotherapy was associated with improved disease-free survival (85.9% versus 77.1%; HR, 0.58; 99.5% CI, 0.41 to 0.82) and overall survival (89.8% vs 86.4%).51,52 In metastatic settings, olaparib was associated with an improvement in progression-free survival (7.0 vs. 4.2 months; HR, 0.58; 95% confidence interval, 0.43 to 0.80) compared to other chemotherapy agents.53
Abemaciclib is a CDK4/6 inhibitor that demonstrated an improvement in disease-free survival of 7.6% at five years in patients with high-risk hormone-receptor-positive breast cancer when treated after standard (neo)adjuvant chemotherapy and surgery. Treatment with two years of abemaciclib improved disease-free survival (DFS) (83.6% vs 76%; HR, 0.68; 95% CI, 0.60 to 0.77).54 Other drugs in the same class include palbociclib, which did not show any benefit in a similar setting, while the data for ribociclib remains immature as of March 2024.55–57 However, early data for ribociclib is promising (3-y DFS rates, 90.4% vs 87.1%; HR, 0.748; 95% CI, 0.618-0.906; P = 0.0014).57
In the metastatic setting, first-line treatment includes a combination of CDK4/6 inhibitor (palbociclib, abemaciclib, or ribociclib) and an aromatase inhibitor (anastrozole or letrozole), which has been shown to nearly double progression-free survival in various trials compared to an aromatase inhibitor alone.58,59,60 The optimal second-line treatment is currently being investigated, with several options available, including fulvestrant, exemestane/everolimus, capivasertib/fulvestrant, elacestrant, alepelisib/fulvestrant, and conventional chemotherapeutic agents. In this setting, molecular alterations in certain genes (PI3K/PTEN/ESR1) can assist in choosing the most appropriate therapy (capivasertib and elacestrant, respectively). However, neither of these drugs are currently available in India.
Immunotherapy: Pembrolizumab, an immune checkpoint inhibitor, inhibits PD-1 and has been shown to improve outcomes in patients with early TNBC and a subset of patients with metastatic TNBC. In the KEYNOTE-522 study, the addition of pembrolizumab to neoadjuvant chemotherapy and its continuation to complete one- year treatment improved disease-free survival to 84.5% compared to 76.8% in the placebo group (0.63; 95% CI, 0.48 to 0.82).61 In the advanced setting, the KEYNOTE-355 trial, it demonstrated that pembrolizumab combined with to chemotherapy in a subset of patients with TNBC (combined positivity score of 10 or more), improved the median overall survival to 23.0 months compared to 16.1 months in the placebo– chemotherapy group (HR, 0.73; 95% CI, 0.55 to 0.95; P=0.0185).
Older frail patients: In older, frail patients with ER-positive breast cancer who cannot tolerate chemotherapy, aromatase inhibitors can be offered, and those with HER2-positive cancer are treated with targeted therapies (trastuzumab with/without pertuzumab). Triple-negative breast cancer in such a population is the most difficult to treat, and these patients are often offered low-dose oral metronomic therapy.62
Radiotherapy: The World Health Organization (WHO) recommends one linear accelerator (linac) per million population.63 With an estimated total population of 1428.4 million in India in 2023,64 according to the WHO standards, India requires 1428 linacs/RT units. As of September 2023, there are 607 radiotherapy centers licensed by the Atomic Energy Regulatory Board (AERB) in India.65 In total, there are 954 RT machines (736 linear accelerators, 174 telecobalt units, 33 Tomotherapy units, and 11 Cyberknife units) [personal communication from AERB]. Most of these facilities are in the private sector and located in urban or semi-urban areas; making them inaccessible to those living in rural areas. Many publicly funded hospitals either lack machines or have limited numbers, which are mostly tele-cobalt units and are incapable of supporting advanced treatment techniques. As a result, there are long waiting lists for treatment, which leads to poor outcomes for patients due to logistic reasons. A simulation model estimated that increasing access to radiotherapy can potentially increase global 5-year breast cancer survival by 1.5% globally, of which LMICs such as India may benefit most possibly seeing gains of 5.8%.66
Most breast cancer patients receive RT using teletherapy (external beam radiotherapy) techniques. For effective breast cancer radiotherapy, minimal, albeit quality- assured infrastructure is essential. The bare minimum should include the availability of:
-
a)
A written standard operating procedure (SOP) detailing the indications for radiotherapy, including communication with surgeons regarding a standard policy for applying tumor bed clips following conservation surgery and, preferably a multidisciplinary forum to discuss all patients requiring radiotherapy.
-
b)
A detailed SOP for planning scan acquisition or a simulator- based radiotherapy field placement is needed.
-
c)
A dose calculation and optimization protocol should be established, incorporating policies using 2-D and 3-D planning techniques. If a 3-D technique is utilized, compatible treatment planning software for the center’s linear accelerator or telecobalt unit is necessary. A select few patients may require inverse planned treatment, and centers needing to commission inverse planned techniques must adhere to the as low as reasonably achievable (ALARA) principle of radiotherapy that the organs at risk doses (heart, lung, contralateral breast, brachial plexus) should be as close as possible to doses achieved using 3-d conformal techniques. Centers should collaborate to peer review the dose-volume constraints and aim for standards established in clinical trials before commissioning any intensity-modulated radiation therapy (IMRT) technique for service.
-
d)
A peer review process of all plans and dose calculations should be encouraged within all treatment centers
-
e)
Centers should be encouraged to participate in national and international studies or projects to facilitate external peer review of breast cancer plans. This is particularly important in the current era with ultra-hypofractionation techniques available for selected patients, where sub-optimal treatment plan leads to significant adverse effects, including breast pain, ultimately impacting patients’ quality of life.67 A major concern in our country is the heterogeneity of the treatment processes and the lack of uniform radiotherapy (RT) quality assurance standards. Quality assurance checks are necessary not only for the equipment but also for generating breast cancer RT plans and treatment delivery. Unacceptable long-term morbidity will affect the patients’ quality of the life and their productivity in society.
Breast cancer RT involves daily sessions lasting from 3 to 6 weeks. This can be a deterrent for patients and caregivers due to the potential loss of income, as they must stay close to the hospital during that time, albeit temporarily. A three-week regimen for whole breast/post-mastectomy radiotherapy is now widely accepted as a standard of care. This has reduced patient’s out-of-pocket expenses for staying away from home. Ongoing research studies are testing the efficacy and safety of shorter (one-week) regimens.68
Skilled manpower is essential for the safe operation of any RT facility. radiation oncologists (ROs), medical physicists (MedPhys), and radiation therapy technologists (RTTs) form the trio that is essential for any radiotherapy center. No formal estimate was available for the number of ROs, Med Phys, or RTTs in the country. There are about 5000 qualified ROs for an approximate annual incidence of 1.4 million new cancer cases, and nearly 600 ROs graduate annually.69 Daphtary et al. (2014) published a study on human resource requirements for cancer control in UP, India , estimating the number of ROs, MedPhys, and RTTs as 5, 4, and 12, respectively, for 1000 new cancer patients.70 Corresponding numbers recommended by the International Atomic Energy Agency (IAEA) are 3-4, 2-3, and 6-7, respectively.71 Most specialized personnel for radiotherapy are concentrated in urban areas where RT infrastructure is available. Unemployment and job saturation have led to qualified ROs moving to obtain Doctorate of Medicine (DM)/Doctorate of National Board (DrNB) Medical Oncology or taking up jobs in general medical service fields that do not treat cancer patients.
Current budget
The sources of funding include:
Government funding
The Indian government allocates funds for cancer prevention, diagnosis, treatment, and research through various national health programs and schemes. This includes the NPCDCS, which aims to strengthen cancer control efforts across the country. Funding is also channeled through government-run healthcare institutions, research organizations, and academic institutions involved in cancer research and treatment.
The Ayushman Bharat Pradhan Mantri Jan Arogya Yojana scheme initiated in 2018 provides funding and has transformed healthcare affordability for patients.72 However, the penetration of the scheme among the underprivileged is less than optimum. This may be due to a lack of awareness and limited access to treatment.
Several State governments are also assisting with cancer treatment, including radiotherapy. The PMSSY scheme provides funding for enhancing/setting up oncology departments in all the AIIMSs and government Medical Colleges.
The government of India has allocated funds to improve facilities in tertiary cancer centers under the SCI and TCCC scheme. Various state governments also provide funding for the purchase of RT machines. The Assam Cancer Care Foundation, a joint venture by government of Assam and Tata Trusts to build 17 cancer hospitals in Assam, exemplifying private-public partnerships to enhance cancer care in the region. Additionally, various not-for-profit charitable hospitals are also available.
Private donations and philanthropy
NGOs, charitable foundations, and philanthropic individuals play a significant role in funding breast cancer awareness campaigns, screening programs, treatment facilities, and patient support services. Several NGOs and advocacy groups raise funds through donations, fundraising events, and corporate partnerships to support their initiatives in breast cancer education, early detection, and patient care.
International collaborations
International organizations, bilateral aid agencies, and global health initiatives collaborate with Indian counterparts to provide funding support for breast cancer control programs, research projects, and capacity-building activities; collaborations with international research institutions and universities often involve funding for collaborative research projects, clinical trials, and training programs for healthcare professionals.
Corporate social responsibility (CSR)
Many corporate entities in India allocate funds for breast cancer initiatives as part of their CSR activities. This includes sponsoring awareness campaigns, organizing screening camps, providing financial assistance to patients, and supporting research projects. Corporate partnerships with healthcare organizations and NGOs are crucial role for expanding access to breast cancer screening and treatment services, especially in underserved communities.
It is equally important to secure funding for radiotherapy research to ensure safer and optimized breast cancer treatment. Currently, there are very few funded options to allow ongoing peer review and quality assurance checks. Initiatives from the government and NGOs to promote peer review and benchmarking will enhance and sustain the quality of treatment delivery. Breast cancer ultra hypofractionation is likely to be adopted in the near future across cancer centers in India. It is essential that quality of treatment is ensured prior to such adaptation to prevent accidents.
Investment in high throughput pathology laboratories in the country is primarily commercially driven, and most cancer centers still struggle to maintain the standard of care. Initiatives like the department of health research (DHR)- Indian Council of Medical Research (ICMR) advanced molecular oncology diagnostic services (DIAMOnDS) project have sought to improve the availability of molecular testing in cancer centers in India, but such a program does not exist for immunohistochemistry. Consequently, since most patients cannot afford their biomarkers, testing services often use cheaper alternatives, while the pathology of paying patients is sent to larger centers, resulting in variable out-of-pocket expenses for patients.
RECOMMENDATIONS
Key issues/gaps identified from public health perspective
Supply side barriers
There are several supply-side barriers to cancer care in India, including:
Skewed geographical distribution of cancer treatment facilities: There is a skewed distribution of cancer care facilities in India, with many areas facing a shortage of services. The number of cancer care facilities is higher in urban areas, especially in major metropolitan cities, whereas rural and remote areas have a limited number of cancer care facilities. This unequal distribution of cancer care facilities can be attributed to a range of factors, including inadequate infrastructure and resources, lack of awareness and education among the population, and insufficient funding and support from the government. In addition, the high cost of cancer treatment and lack of access to affordable healthcare services further exacerbate the problem of unequal distribution of cancer care facilities, particularly for people from economically disadvantaged backgrounds.
The existing evidence suggests that nearly 60% of specialist facilities are located in the southern and western regions of India.29 However, over 50% of the population resides in the eastern and central regions, creating a distortion in service provision. For example, at least half of cancer patients will require radiotherapy at some point. However, data given by the Atomic Energy Regulation Board reveal that 26% of the population residing in India’s eastern area has immediate access to only 11% of radiotherapy facilities [Figures 5 and 6].
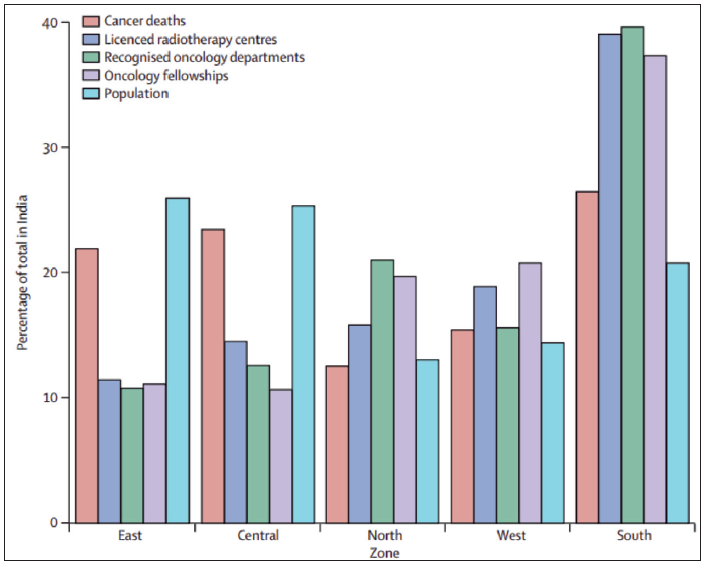
- Population & cancer mortality against the corresponding proportion of cancer care facilities in India. Source: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(14)70115-9/abstract?version%253DprinterFriendly=
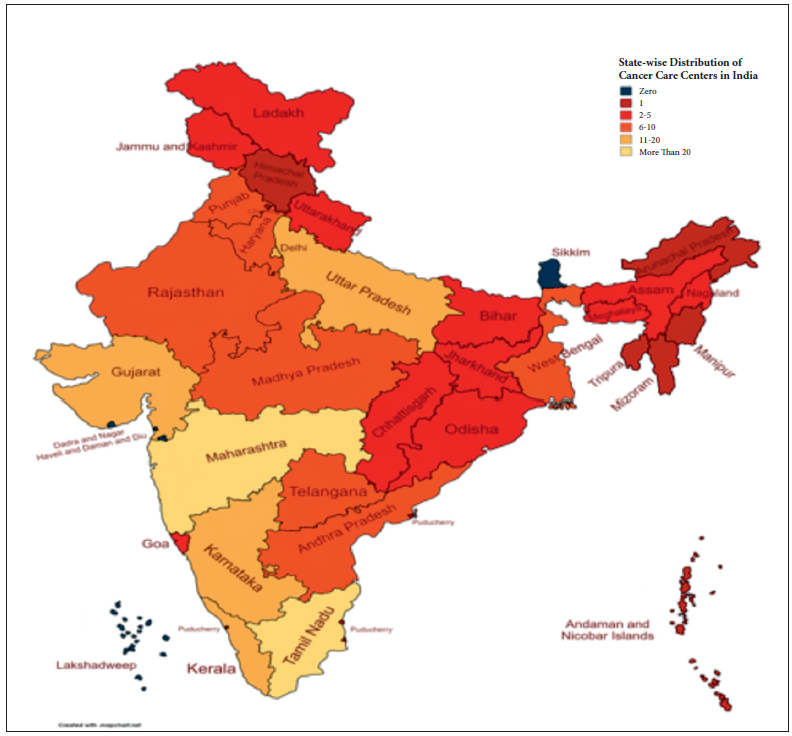
- State-wise distribution of cancer care centres in India. Source: National Cancer Grid, https://www.ncgindia.org/hospitals-and-institutions/members
Lack of treatment facilities: Radiotherapy is an essential component in the management of breast cancer patients and is used in conjunction with chemotherapy or surgery for both curative and palliative purposes. Modern-day cancer care increasingly requires a joint multimodality approach.3 It is estimated that approximately 50% of all cancer patients are cured by surgery, 40% by radiotherapy alone (or combined with other surgery/chemotherapy), and 10% by chemotherapy alone (or combined with other surgery/chemotherapy).3 The treatment of breast cancer requires a multimodal approach using all three modalities.
Present radiotherapy infrastructure in India: The radiotherapy centers in India have either teletherapy facilities alone or both teletherapy and brachytherapy facilities [Table 1]. The Directory of Radiotherapy Centers (DIRAC) 2012 from the IAEA has classified India alongside the poorest Sub-Saharan African countries, which have fewer than one radiotherapy machine per million people73 [Figure 7]. Globally, India has the largest number of people living below the World Bank’s poverty line of US$ 1.25 per day.64 Currently, there are a total of 954 RT machines (736 linear accelerators, 174 telecobalt units, 33 Tomotherapy units, and 11 Cyberknife units) [personal communication from Atomic Energy Regulatory Board (AERB)].
| Region | Population of each region (%) | Area of each region (%) | Simulator | Number of machines available in each region (%) | Cyber Knife | Gamma Knife | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CT-Sim | Telecobalt | Linacs | RAL Brachy | Tomo | ||||||
| Central | 8.10 | 13.6 | 1 (2.5) | 2(4) | 15 (8.3) | 12 (3.3) | 13 (5.2) | 0(0) | 0(0) | 0(0) |
| East | 22.33 | 12.8 | 4(10) | 1 (2) | 20 (11.1) | 22 (6) | 16 (6.4) | 1 (12.5) | 0(0) | 0(0) |
| North | 24.82 | 20.5 | 15 (37.5) | 13 (26) | 42 (23.3) | 85 (23.3) | 65 (26) | 1 (12.5) | 3 (42.9) | 5 (71.4) |
| North-East | 3.57 | 7.8 | 1 (2.5) | 3(6) | 10 (5.6) | 6 (1.6) | 6 (2.4) | 0(0) | 0(0) | 0(0) |
| South | 21.09 | 19.4 | 12 (30) | 18 (36) | 50 (27.8) | 150 (41.1) | 88 (35.2) | 3 (37.5) | 4 (57.1) | 1 (14.3) |
| West | 20.09 | 26.0 | 7 (17.5) | 13 (26) | 43 (23.9) | 90 (24.7) | 62 (24.8) | 3 (37.5) | 0(0) | 1 (14.3) |
| Total | 100 | 100 | 40 (100) | 50 (100) | 180 (100) | 365 (100) | 250 (100) | 8 (100) | 7 (100) | 7 (100) |
States included in each region: Central: Chhattisgarh, Madhya Pradesh, East: Bihar, Jharkhand, Orissa, West Bengal, North: Chandigarh. Delhi, Haryana, Himachal Pradesh. Jammu and Kashmir, Punjab, Uttar Pradesh. Uttarakhand, North-East: Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, Tripura, South: Andhra Pradesh, Karnataka, Kerala, Puduchery, Tamilnadu. Telengana. West: Goa, Gujarat Maharashtra. Rajasthan. States not included: Andaman & Nicobar Islands. Sikkim. D & N Haveli. Daman & Diu. Lakshadweep; CT: Computed tomography, RAL: Remote after-loading
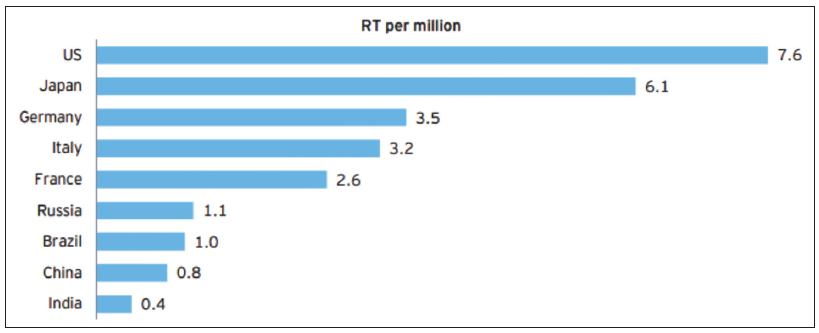
- Availability of radiotherapy equipment across different countries. Source: “World population”, worldpopulationreview.com “Directory of radiotherapy centres”, Dirac.iaea.org. RT: Radiotherapy
Per capita radiotherapy statistics in India: In most high-income countries, there is at least one radiotherapy unit available for every 250,000 people,5 which translates to an average of four radiotherapy machines per million population. Applying this factor to India indicates a total requirement of 5000 radiation therapy units in India now [Table 2]. Based on the current number of installed units in India, this reflects a shortfall of over >4500 machines. According to the WHO, there should be one teletherapy unit for every million people. There would still be a major shortage of teletherapy units in the country [Table 2]. While the number of teletherapy units has increased since this data was published; however, this is still lower than the optimal number.
| RT Equipment/Manpower | Recommendation in West (Per million people) | Required for whole country* | Existing, in the country | Shortfall |
|---|---|---|---|---|
| Teletherapy | 4 | 5000 | 545 | 4550 |
| Simulator | 1 | 1250 | 90 | 1050 |
| TPS | 1 | 1250 | 500 | 750 |
| Brachytherapy (remote) | 1 | 1250 | 250 | 1000 |
| Radiation oncologist | 4 | 5000 | 1000 | 4000 |
| Medical physicist | 4 | 5000 | 1150 | 3850 |
| Radiotherapy technologist | 6 | 7500 | 2200 | 5300 |
Demand side challenges: To understand demand-side challenges or barriers, it is essential to understand them at individual and community levels. Most of the barriers have overlapping levels as these levels have their parts to play while attributing demand-side challenges [Figure 8].74
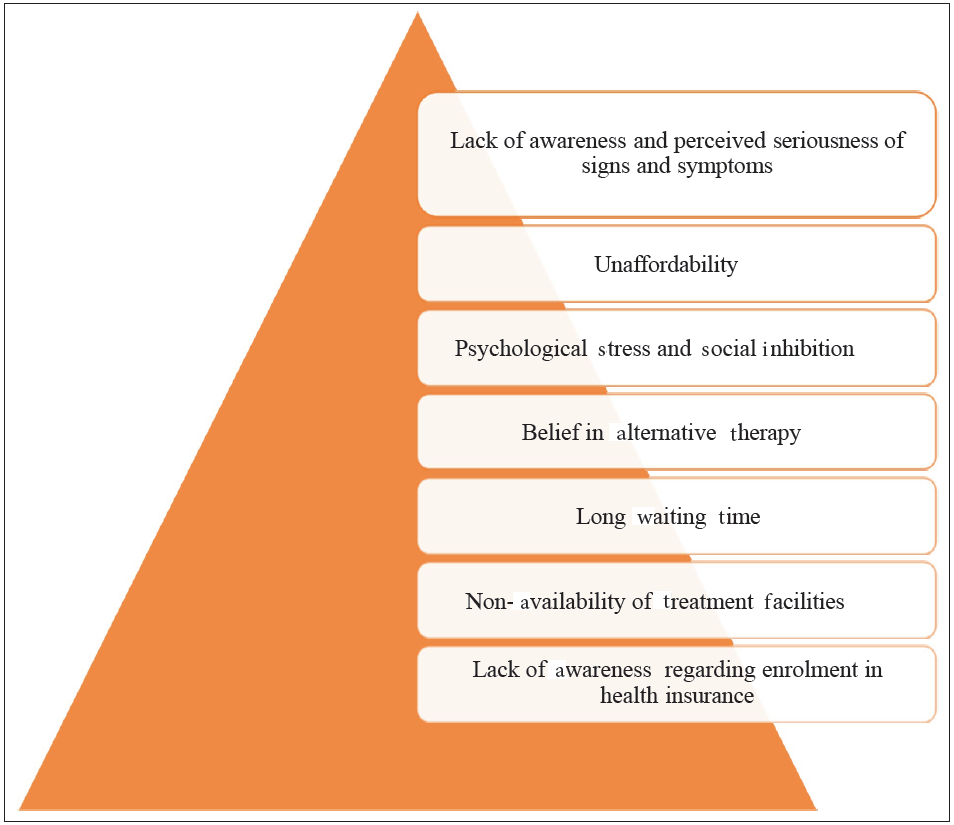
- Demand side barriers in cancer treatment. Source: https://www.sciencedirect.com/science/article/abs/pii/S1877782113001641
At individual level: Although tobacco use, lifestyle choices, and alcohol consumption have been proven to be causes of cancer, impediments related to treatment delays also significantly add to the cancer mortalities burden.75 A systematic review identifying various delay stages in cancer diagnosis confirmed that there are identifiable stages between recognizing a symptom, first presenting to a health care professional, subsequent diagnosis, and initiation of treatment.75 The various reasons for the delay in treatment-seeking behavior at the individual level were:
Lack of awareness and perceived seriousness of signs and symptoms: For early diagnosis and the prompt initiation of therapy, it is crucial to recognize cancer signs and symptoms as soon as possible. A study conducted in Odisha found that most patients had never heard of cancer, and less than one-sixth were aware of carcinogenic factors.74 Low levels of knowledge, educational status, and perception of the illness severity are the leading causes of delay in getting cancer treatment.76,77
Given the lack of awareness and the fact that many cancer symptoms resemble those of other more prevalent benign conditions, it might be difficult for the patients to connect their symptoms to cancer.78 One can argue that these factors could contribute to delayed consultation and prolonged watchful waiting74 [Figure 9].
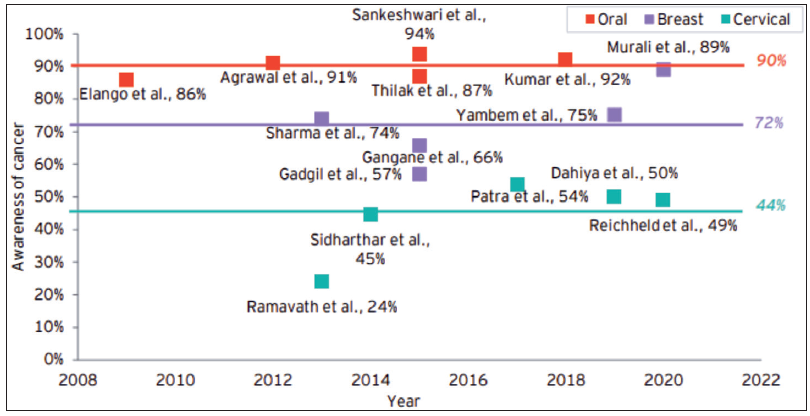
- Awareness among the cancer patients in India. Source: Call for Action: Making quality cancer care more accessible and affordable in India, October 2022 - EY analysis report
Financial constraints: Lower financial capabilities often lead individuals to adopt a more fatalistic and pessimistic when seeking healthcare.79 Patients with low socioeconomic status (SES) tend to be diagnosed with more advanced cancers, receive less aggressive treatment, and face a higher risk of dying in the 5 years of diagnosis.80,81 A study from India reported financial constraints as the major barrier to seeking treatment.74 The analysis of the World Health Survey showed that low-household SES was significantly associated with cervical and breast screening rates in low-income countries.80
At the Individual and Community level
Psychological stress and social inhibition
Negative attitudes, stereotypes, and discrimination toward cancer patients are common in many societies.82,83 Studies have found that cancer patients often experience social inhibition when speaking with community members about their symptoms.73,74 Cancer-related stigma is not only associated with delayed treatment-seeking behavior but also with poor self-esteem, stress, anxiety, depression, and social isolation in different patient groups84,85 [Figure 10].86
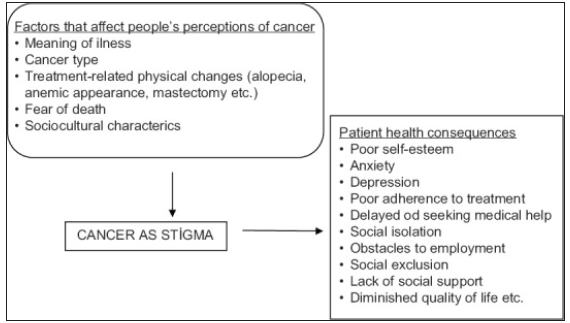
- A conceptual framework of cancer stigma. Source: https://www.sciencedirect.com/science/article/pii/S2347562521001827
A meta-synthesis exploring barriers to health-seeking behavior among Malaysian breast cancer patients indicated that stress and a sense of denial were two prominent psychological factors linked to delays in seeking treatment.87 This suggests that various social and psychological reasons hinder immediate treatment consultation or access to advanced care and surgery.
Belief in alternative therapy: According to estimates, cancer patients spend an average of an additional 139 days consulting various healthcare systems, including traditional healers and alternative medicine practices such as Ayurveda and homeopathy.74 A cross-sectional study in Bangladesh demonstrated that the likelihood of alternative medicine delaying treatment-seeking behavior was four times higher among Bangladeshi Breast Cancer Patients.88 In India, an exploratory study revealed that nearly half of the cancer patients reported being treated with complementary and alternative medicine (CAM).89 Thus, belief in alternative therapies whether due to influenced or due to financial constraints has led to delays in availing conventional cancer treatment90 [Figure 11].74
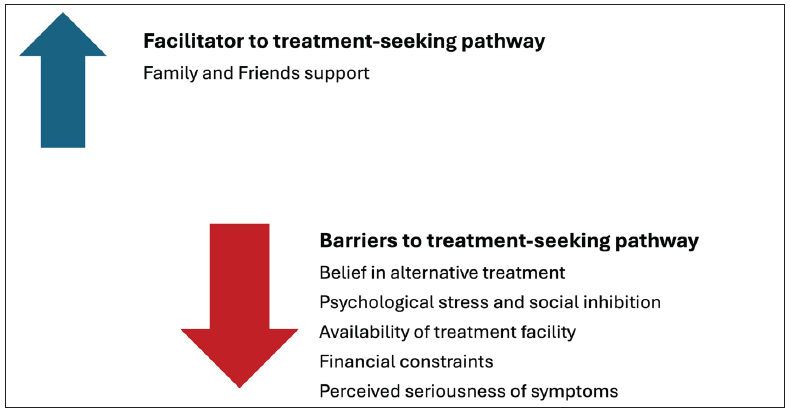
- Qualitative qualifiers affecting the treatment-seeking pathways. Source: https://www.sciencedirect.com/science/article/abs/pii/S1877782113001641
At the individual and health system level
Enrolment in health insurance schemes
In India, the launch of Ayushman Bharat PM-JAY in 2018 marked the introduction of the world’s largest health assurance scheme in the world, making it crucial for the population to be aware of the insurance scheme. The scheme provides health cover of Rs. 5 lakhs per family per year for secondary and tertiary care hospitalization to over 10.74 crores of poor and vulnerable families, accounting for approximately 50 crore beneficiaries in the bottom 40% of the Indian population. However, a study conducted in one of the Indian states in 2021 assessed that awareness about the AB-PMJAY for treating disease, especially cancer care, was only about 50%).91 The lack of awareness can be attributed to many factors, such as illiteracy, ignorance, improper knowledge about the scheme, and poor hospital connectivity. Even after enrolling in the scheme, the above factors lead to the inability to properly utilize the facilities.92 While the government is emphasizing the need for increased awareness, recent studies have reported raising awareness levels among the population. Nonetheless, coverage and utilization of the scheme among the targeted groups remain minimal,92 highlighting the need to enhance demand from the population..
Human resource barriers to access to breast cancer care
While human resources are essential for providing cancer care in India, several challenges impede their effectiveness, as outlined in Figure 12.
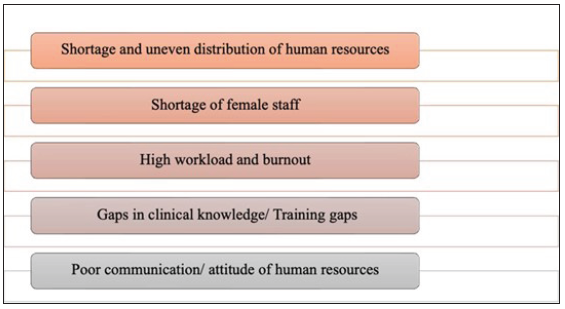
- Human resources-related barriers to access to breast cancer care.
Shortage of skilled personnel: There is a shortage of healthcare professionals involved in oncology care at all levels of care, which limits the capacity to provide timely and comprehensive cancer care, particularly in rural and underserved areas. India exhibits a shortage of oncologists, with 2000 oncologists for 10 million patients, which is further skewed toward urban areas.16,29,93 Additionally, there is a notable north-south divide in availability, with about 60% of specialist facilities and the workforce located in southern and western India. Figure 13 depicts the shortage in demand and supply of treatment providers for cancer care.93
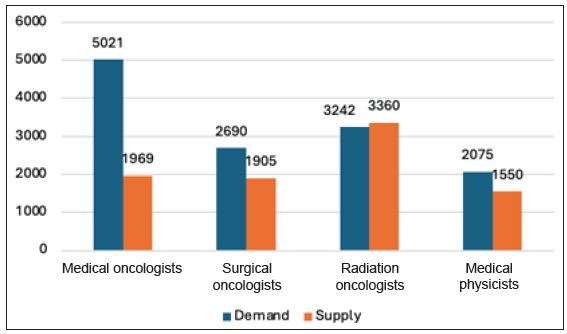
- Difference in demand and supply of human resources.
Furthermore, it has been noted that 27% of CHCs and 13% of district hospitals had not implemented the national program for control of non-communicable diseases in 2017- 2018 due to a lack of workforce or equipment necessary for diagnosing cancers. This is in line with the general perception of primary care physicians on the limited utility of referrals to secondary-level health centers due to a shortage of specialists and equipment for confirmatory diagnosis and staging.
Shortage of female staff: The scarcity of female doctors, particularly in the context of breast cancer care, presents additional challenges to healthcare delivery in India. Several studies have reported the unavailability of female staff acts as a barrier to accessing breast cancer-related care due to cultural, religious, and social values.94,95 The lack of female healthcare providers has been found to create barriers to open communication, leading to delays in seeking care, reluctance to undergo screenings, and decreased adherence to treatment recommendations.
High workload and burnout: The burden of cancer care in India is notably high, with each oncologist facing a staggering workload of 315 cases per practitioner, significantly surpassing the workload per oncologist in countries like China and the United States [Figure 14].96 This overwhelming demand for medical and surgical oncologists exceeds the current availability by 2.6 and 1.4 times, respectively.93 Consequently, skilled professionals experience immense pressure, impacting the quality of care, timely diagnosis, and patient attrition rates.
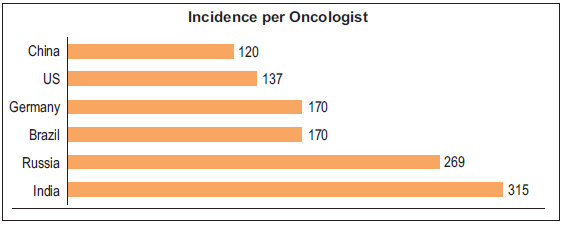
- Comparison of incidence per clinical oncologist (radiation + medical). Source: https://ascopubs.org/doi/full/10.1200/JGO.17.00188
Research has elucidated various factors contributing to this strain on the healthcare system. For instance, studies have revealed that auxiliary nurse midwives (ANMs) in India, who play crucial roles in primary healthcare delivery, are often burdened with multiple responsibilities under national healthcare programs during scheduled work hours.97 This situation results in the neglect of cancer care services, as resources are diverted to address other pressing healthcare needs.
Furthermore, investigations into specialized cadres of medical oncologists from LMICs, including India, have underscored the alarming volume of work undertaken by these professionals.98 The median number of annual consultations per medical oncologist in India stands at 475, significantly higher than the corresponding figure in other LMICs, which is around 350. Such high caseloads inevitably lead to burnout among healthcare staff, impacting their well-being and further straining the healthcare system’s capacity to deliver quality cancer care.
Lack of adequate training in the workforce: Since breast cancer screening tests are dependent on observation, they have a high reliance on the skill set of the healthcare provider. Therefore, it is crucial to invest in the training of healthcare professionals to ensure effective screening. Of the current workforce deployed at HWCs nationwide, 26% still require training to perform screening tests. Among the ASHA workers, who form the backbone of the public health program in India, at least 23% need training for screening NCD patients, including those with malignancies. Additionally, nearly 16% of the 2,761 medical officers stationed in these facilities require training on the screening program and techniques [Figure 15].99
- Percentage of trained health and wellness centre staff for prevention, screening, and management of NCDs, including cancer. Source: https://europepmc.org/article/nbk/nbk525285. NCDs: Non-communicable diseases, MPW-W: Multipurpose worker-women, MPW-M: Multipurpose worker-Men, ASHA: Accredited social health activist.
Gaps in training and continuing medical education: The failure of healthcare providers to recognize the signs and symptoms of breast cancer and to consider it as a potential diagnosis has emerged as a significant barrier to the timely detection and treatment of this disease. Studies investigating the knowledge, attitudes, and practices of healthcare providers across various cities have revealed low levels of awareness regarding breast cancer screening, available methods, and the practice of self-breast examination. For instance, research conducted in northern India uncovered a lack of awareness among 49% of healthcare workers regarding the risk factors and early detection methods associated with breast cancer.100 These findings underscore the critical need for capacity-building initiatives and ongoing medical education to bridge these knowledge gaps.
Furthermore, studies have highlighted deficiencies in capacity-building efforts, with one-fourth of the staff at Ayushman Arogya Mandirs, healthcare facilities providing primary care services, lacking formal training. An Indian study reported the median time to diagnosis from the first contact with a provider as 30 [IQR 10-60] days, majorly due to a lack of awareness in identifying the cancer symptoms both among patients and primary care providers.101 Thus, these gaps in training and education contribute to delays in the diagnosis of breast cancer, hindering efforts to identify and treat the disease at an early stage when outcomes are typically more favorable.
Poor doctor-patient communication: Effective communication between physicians and patients is crucial, as it has been consistently linked to positive health outcomes, including increased patient satisfaction, compliance with treatment plans, and overall improvement in health status. However, studies have revealed that patient-doctor communication in South Asia is often perceived as dissatisfying by patients, primarily due to a predominant pattern of one-way communication by healthcare providers.94,95 The literature highlights that this deficiency in communication has significant implications for the accuracy and timeliness of diagnosis and treatment of breast cancer, highlighting the role of health system factors in shaping patient experiences and outcomes. While some studies have noted instances of comforting behavior exhibited by doctors, concerns have been raised regarding the attitude of paramedical staff and hospital administration.102 Negative interactions with these healthcare professionals further exacerbate patients’ dissatisfaction and impede their ability to navigate the complexities of breast cancer diagnosis and treatment.
Training of manpower remains an Achilles’ heel in our ever-expanding cancer service strategy. More centers should be encouraged to establish in-house training for radiation oncologists, medical physicists, and radiotherapy technicians.
The current training in Radiation Oncology is short and quite generalized. Promoting site specialization in breast cancer oncology is essential for improving quality. Site-specific (e.g., breast cancer) Certificate courses or fellowships in high-throughput regional cancer centers must be encouraged and officially recognized. Additionally, international exposure should also be encouraged to understand the safeguards inherent to starting clinical practices. Hypofractionation and use of regional nodal irradiation remain topical issues, and mandatory seminars and certification should be encouraged to allow the same implementation.
Centers should be incentivized to start medical physics training and internship programs. Clinical site specialization of medical physicists during internship could be offered given the complex treatment processes. Radiotherapy quality assurance, junction dosimetry, as low as reasonably achievable (ALARA) principles, and its implementation in breast radiotherapy must be encouraged so that cardiac, lung, and brachial plexus doses do not compromise patient safety during breast radiotherapy.
Radiotherapy technicians must be well versed in the implementation of all treatment types and image guidance processes and also flag to clinicians any on-treatment adverse events. This, therefore, means that they are practically well-trained to ascertain when toxicities are more than envisaged. A career pathway for bright radiographers to be upgraded to dosimetrists after bridging courses are established could help address both skill upliftment issues and fulfill the need for more radiotherapy planning stuff by all departments.
Communication skills training is important across staff categories, and regular programs should organize regular programs to ensure that the benefits, risks, and both short- and long-term adverse events are communicated appropriately and in detail to patients.
Other barriers
Inadequate population coverage under cancer prevention and screening programs: There is a lack of cancer prevention and screening programs in India, which can lead to delayed diagnosis and treatment. This can result in poorer outcomes for cancer patients. The national cancer screening program in India has been in operation since November 2016. However, population coverage for cancer screening has been extremely low. Since 2018, when cancer screening was included in the National Health Mission’s larger NCD screening program, India has made some progress. To date, 1.1% of the population has undergone cervical cancer screening, and less than 1% have received breast or oral cancer screening. Due to easy access to screening facilities, opportunistic screenings in private hospitals, and increased public awareness, screening coverage in urban regions is marginally better than that in rural ones. However, coverage for oral cancer screening among men in both urban and rural areas is low [Figure 16].
- Screening coverage in India by percentage of population.
Due to inadequate screening systems and low awareness, late-stage disease identification exacerbates the problem of a high disease burden. Only 29% of breast cancers are detected at stages 1 and 2, respectively, India has a poor detection rate across major cancer sites, which is much lower than that in China, the UK, and the US [Figure 17]. This low rate of early diagnosis contributes to India’s high mortality rate for malignancies, especially when compared to industrialized nations like the US and the UK, where early detection has been crucial in reducing mortality.
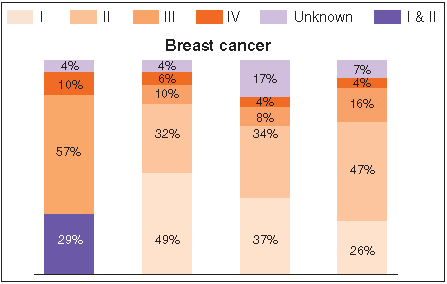
- Percentage of cases diagnosed at various stages of breast cancer.
High cost of cancer treatment: The high and increasing cost of healthcare presents a major public health challenges in India. The amount of impoverishment and debt caused by high out-of-pocket expenses (OOPE) is growing as households continue to be the main source of funding for healthcare. Significantly, the OOPE for people with cancer is 2.5 times higher than for those with other disorders. The cost of cancer treatment can drive individuals and families into severe misery and even insolvency. Even Although the cost of treating cancer patients in hospitals is the highest among all NCDs, ineffective health financing systems and a heavy reliance on out-of-pocket medical expenses force many cancer patients to resort to desperate measures to afford their treatment.103
One of the studies assessing the financial toxicity associated with cancer treatment reported that the prevalence of catastrophic health expenditure due to outpatient treatment was 80.4%, while the prevalence due to cancer-related hospitalization was 29.8%. The overall prevalence of impoverishment was 67% as a result of outpatient cancer treatment and 17.2% due to hospitalization [Figure 18].104
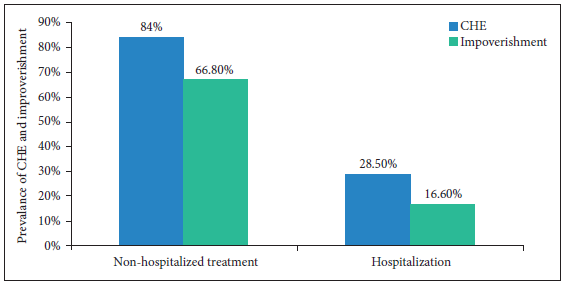
- Financial toxicity due to cancer treatment in India. Source: https://www.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2023.1065737/full, CHE: Catastrophic health expenditure.
Inadequate health insurance coverage: Inadequate health insurance coverage can limit their ability to access cancer care services [Figure 19].104
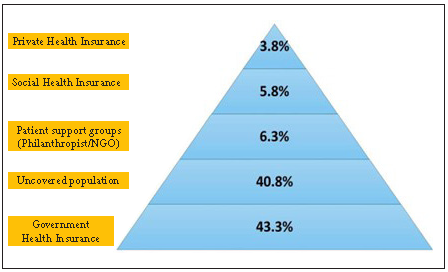
- Coverage of health insurance in India. Source: https://www.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2023.1065737/full, NGO: Non governmental organization.
Overall, these barriers to cancer care in India highlight the need for improved infrastructure, increased availability of healthcare professionals and cancer drugs, and expanded access to health insurance and cancer prevention and screening programs.
To implement the NPCDCS program under the auspices of universal primary health coverage, the government has included and prioritized screening for the three cancers as part of the program and is working to meet the goal of opening 1,50,000 health and wellness centers (HWCs) throughout India by December 2022. However, several challenges exist that are impacting the screening program from achieving its desired objectives [Table 3].
| Indicator | Target | Status | Key points |
|---|---|---|---|
| Capacity - Physical infrastructure | 1,50,000 HWCs with the necessary infrastructure required for screening of cancer within 30 minutes distance of the covered population by December 2022 | As of March 2022, 1,17,000 HWCs set up | It is still difficult to find an appropriate number of HWCs nationwide. Moreover, some of the HWCs that are currently in operation lack a specialised area for cervical or breast examinations, considering privacy and infection control regulations. |
| Capacity - Workforce availability | According to IPHS guidelines, each sub-centre should be staffed with 1 CHO, 5 ASHAs, 2/3 multipurpose workers and PHC with 1 medical officer and other staff. | As of March 2021, there is shortage of 2.9% of female health worker/ ANM primarily due to shortfall in Himachal Pradesh, Rajasthan, Gujarat, Tripura, and Kerala. In comparison to those proposed at the PHC level, there is a shortfall of medical officers of around 4%, primarily in Orissa, Karnataka, and Chhattisgarh. | Having a skilled and sufficient workforce to cover the population for the HWC is as crucial to the physical infrastructure. Due to social and privacy concerns, a lack of female health professionals has a direct impact on the screening coverage for breast and cervical cancer screening. Any screening program’s performance is further hampered by higher referral centres’ insufficient staff, which delays confirmed diagnoses and, consequently, treatment, contradicting the purpose of early detection. |
| Capacity– Training | Every employee at HWCs has received cancer screening training. | There are currently 23% untrained employees working in these HWCs. | Conducting screening is quite difficult with an untrained team. A successful screening campaign is not always guaranteed by the simple availability of manpower. |
| Capacity- Referral network | Refer all cases ‘at risk’ to higher centres | Till 2017-18, NPCDCS had not been implemented in 27% of CHCs and 13% of DHs. | Triaging patients and sending them to the proper referral facilities is one of the key functions of health and wellness centres. The difficulty lies in these referral hospitals’ readiness to take these patients. Less than 10% of the facilities at the district hospital where NPCDCS was implemented by the government had all the equipment for cancer screening. |
| Awareness | The objective is to raise awareness of cancer screening among all people through various information, education, and communication strategies. | According to several studies, healthcare professionals and the general public have little awareness about cancer screening. | Awareness, attitude, and knowledge, toward screening of cancer become important in a national screening program |
| Affordability and financing | At government centres, screening and treatment post diagnosis for the patients is free of cost. | About 55% patients with cancer are required to rely on private hospitals for management. Ayushman Bharat’s and state healthcare programs had provided limited co verage, along with 10% to 12% of coverage by private insurance. | The financial condition of people affected with cancers is significantly impacted among all NCDs as cost of cancer treatment is highest. |
HWC: Health and Wellness Centre; CHO: Community Health Officer; ASHA: Accredited Social Health Activist; ANM: Auxiliary Midwife Nurse; NPCDCS: National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases, and Stroke; DH: District hopital, NCDs: Non-communicable diseases
Recommendations made to bridge the critical gaps/ deficiencies in public health perspective
Recommendations to close the human resource gaps for cancer care
Closing the human resource gaps for cancer care requires a comprehensive and multi-dimensional approach that addresses workforce shortages, enhances training and education, and fosters professional development. We propose the following recommendations [Figure 20] to strengthen the health system building block of human resources to ensure access to quality cancer care:
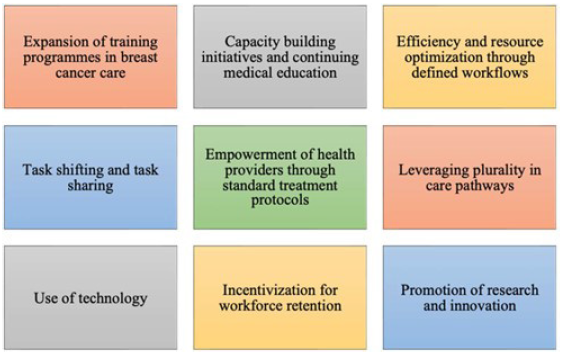
- Recommendations to close the human resource gaps for cancer care.
Expansion of training programs in key areas of breast cancer care: The chronic shortage of staff for the provision of oncology care demands investment in expanding the capacity of medical schools, nursing programs, and allied health training institutions to produce more healthcare professionals specialized in key areas of cancer care, such as medical oncology, surgical oncology, radiation oncology, oncology nursing, palliative care, and supportive care.93,105 This could involve increasing the number of training slots, establishing new training programs, or offering scholarships and incentives to attract students to oncology-related fields. Furthermore, such programs should focus on both clinical and non-clinical aspects of cancer care, including communication skills, patient-centered care, and interdisciplinary collaboration.
Capacity-building initiatives and continuing medical education: Structured capacity-building sessions based on comprehensive programs that cover various aspects of cancer care, including prevention, screening, diagnosis, treatment, and palliative care, should be organized for existing healthcare professionals to enhance their skills and knowledge in cancer care.106
Healthcare professionals should be provided with a variety of continuing education opportunities, including workshops, seminars, conferences, webinars, and online courses that focus on emerging trends, best practices, and evidence-based guidelines in cancer care. These activities should be accredited and provide healthcare professionals with continuing medical education (CME) credits.
Efficiency and resource optimization through defined workflows: Interdisciplinary teamwork plays a pivotal role in improving patient outcomes and ensuring holistic care for individuals affected by cancer. By fostering collaboration among healthcare professionals from diverse disciplines, including oncologists, surgeons, nurses, social workers, psychologists, rehabilitation specialists, and community health workers, healthcare systems can effectively address the complex needs of cancer patients across the continuum of care.106,107 One key aspect of fostering interdisciplinary teamwork is the development of clear guidelines and algorithms for service delivery, particularly in the context of breast cancer care.
Task shifting and task sharing: India’s structured workforce of primary care professionals and community health workers presents a valuable resource for expanding access to cancer care, particularly in resource-constrained settings. By leveraging the skills and capabilities of non-physician healthcare providers, such as nurse practitioners, physician assistants, community health workers, and peer educators, tasks and responsibilities can be delegated effectively under appropriate supervision and training. Task-shifting and task-sharing strategies can optimize healthcare delivery efficiency and improve access to cancer care services, especially in communities where access to specialized healthcare facilities is limited.
Drawing inspiration from successful models implemented in other countries, such as Peru, we can explore innovative approaches to community-based cancer care. For instance, Peru developed a model that trained community health promoters for outreach, professional midwives for clinical breast examinations, doctors for fine- needle aspiration biopsy sampling with ultrasound for triage, and patient navigators to ensure treatment adherence.108 This holistic approach effectively addressed accessibility barriers and improved outcomes for the population.
Moreover, evidence from a 20-year prospective cluster-randomized trial conducted in Mumbai underscores the potential impact of task-shifting in cancer care. Primary health workers, with a minimum educational qualifications of matriculation, were trained to perform clinical breast examinations every 2 years. This intervention resulted in a reduction in the staging of breast cancer at diagnosis and a significant decrease in mortality among women aged 50 years and older.109 This demonstrates the feasibility and effectiveness of training non-specialized healthcare workers to conduct essential cancer screening activities within a relatively short training period.
Empowerment of health providers through standard treatment protocols: Standard treatment protocols empower healthcare providers in decision-making while providing care to patients with breast cancer. Studies highlight that healthcare providers perceive the lack of standard treatment protocols as a hurdle in the provision of care for breast cancer in the communities. Therefore, it is essential to ensure access to defined protocols and necessary training covering all aspects of care, including screening, diagnosis, staging, treatment modalities (such as surgery, chemotherapy, radiation therapy, and targeted therapy), follow-up care, and supportive care interventions.
Furthermore, standard treatment protocols should be regularly reviewed and updated based on emerging evidence and advancements in breast cancer research and treatment. Healthcare providers should be involved in the review process to provide feedback and insights from their clinical experiences, ensuring that the protocols remain relevant and effective in addressing evolving challenges in breast cancer care.
Leveraging plurality in care pathways: Studies have shown that a significant proportion of patients with breast cancer utilize traditional, complementary, and alternative medicine (TCAM) systems.109,110 Therefore, the TCAM providers should be linked to the formal healthcare system to ensure standard, timely care to the patients. Adequate training should be ensured for the TCAM providers to ensure they have a comprehensive understanding of breast cancer, including its diagnosis, treatment modalities, and potential interactions with TCAM therapies. This training should emphasize evidence-based practices and promote safe and effective integration of TCAM with conventional cancer care. Additionally, structured pathways for integrative care that outline clear guidelines and protocols for collaboration between TCAM providers and formal healthcare professionals should be developed.
Use of technology: Leveraging telemedicine and teleconsultation platforms in cancer care can revolutionize access to specialized services, especially in remote and underserved areas of India. By connecting cancer patients with oncology specialists regardless of geographical location, these platforms address the shortage of oncologists and improve access to timely and quality care.111,112
In addition, the emergence of artificial intelligence (AI)-based technologies for breast cancer screening offers a promising solution to the challenges of early detection, particularly in regions with limited access to specialists and cultural barriers. Technologies such as iBreastExam, Mammoassist, Niramai, and Mammoalert use AI algorithms to detect abnormalities indicative of breast cancer.112 Studies have shown that untrained healthcare workers can effectively use these AI-based devices with minimal training, achieving greater sensitivity than traditional clinical breast exam methods.
Incentivization for workforce retention: Policies and incentives should be implemented to attract and retain healthcare professionals in cancer care, including career advancement opportunities, workload management strategies, financial incentives, recognition, and support for work-life balance.
Promote research and innovation: It is essential to encourage research and innovation in cancer care workforce development, including studies on workforce needs, best practices in training and education, and novel models of care delivery. These studies can help inform workforce planning and resource allocation strategies to ensure that the healthcare system is equipped to meet the growing demands for cancer care services. Research efforts should focus on evaluating the effectiveness of existing training and education programs for healthcare professionals involved in cancer care. Studies should assess the impact of different training modalities, curriculum content, and teaching methods on knowledge acquisition, skill development, and clinical practice outcomes. By implementing these recommendations, healthcare systems can address human resource gaps in cancer care, strengthen the capacity of the workforce, and improve the quality and accessibility of cancer care services for individuals affected by cancer.
Recommendations for addressing the demand side barriers
The recommendations are classified as immediate-term, medium-term, and long-term to address this gap.
1. Immediate-term (1-2 years)
-
(i)
The integrative approach: A multidisciplinary approach integrating traditional medical practices with modern medicine may be considered, where unequivocal evidence exists.113 For example, a recent report summarized evidence and cost-effectiveness of diet and physical activity in primary prevention of breast cancer.114 Such practices may be integrated in clinics.
-
(ii)
Empowering cancer awareness through the internet: Regular education programs adopting the specific cultural contexts of local communities have led to an increased participation in screening programs.114 Awareness about breast cancer in general population has to be increased substantially, and opportunistic education of relatives of patients is one of the effective measures. However, this may not always be possible at high-volume government centres in India. An alternative measure includes distribution of printed pamphlets that provide reliable and valuable information. With the increasing number of internet users, web content is increasingly being employed to disseminate health-related information.115,116 Online search for information has become accessible to the population. However, this is a double-edged sword with a plethora of misinformation being readily available and therefore, Therefore, credible information sources, like ‘Cancer India,’ has been developed by the National Institute of Cancer Prevention and Research, that provides authentic and comprehensive source to information.117 The website has succeeded in providing the intended information to the target population.118
2. Medium term (3-5 years)
Digital breast tomosynthesis and newer biopsy techniques are available to diagnose breast cancer at an early stage.16 These innovative health technologies can be evaluated through cost-effectiveness analysis to identify the best diagnostic and therapeutic interventions offering maximum value for money.
-
(i)
Affordability and financing: Designing an intervention to provide financial risk protection against outpatient treatment: A recent study104 reporting financial toxicity related to cancer indicated that the high economic burden of cancer treatment arises primarily from outpatient care rather than hospitalization . This finding significantly impacts existing publicly financed health insurance schemes which do not cover outpatient cancer care. An important recommendation of the study is to design interventions to provide financial risk protection against outpatient treatment.
-
(ii)
Strengthening the public sector: Cancer care delivery in public sector hospitals is more cost-effective, and therefore, strengthening delivery in public sector is likely to prevent financial toxicity. Furthermore, publicly financed insurance is also more likely to provide protection against the financial risks. Such benefit packages should be rationally expanded to provide more cost effective treatments to a larger population, thus moving towards the goal of universal health coverage.
-
(iii)
Digital solutions for mitigating out-of-pocket expenditure on diagnostics: An important determinant of OOPE for outpatient care is diagnostics accounting for 36.4%, as reported in a recent study by Prinja et al. (2023).104 Digital technological solutions may play an important role here. The Government of India recently launched a digital voucher to provide social assistance on subsidy. This digital voucher, E-RUPI,9 can be used to pay for expensive diagnostic and staging investigations once a patient has been confirmed to have cancer following histopathology.. A pilot program for this application of E-RUPI has been planned by the AB PM-JAY. Additionally, more laboratory and diagnostic centers are becoming part of the digital health ecosystem under the Ayushman Bharat Digital Mission (ABDM), enhancing the effectiveness of these initiatives.
Digital solutions for mitigating out-of-pocket expenditure on medicines
Expansion of E-RUPI can be used to pay for medicines in outpatient settings, which have been shown to constitute 27.8% of the total OOPE.104,119
The government should also nudge private insurance providers to include cancer screening in their plans.
-
(iv)
Infrastructure and capacity restraint: The funds should be used not only to establish all 1,50,000 HWCs but also to ensure that these facilities employ adequately educated staff, including trained women, to inform the public about cancer screening and early warning indications.
A financial incentive system should be implemented at the local level to encourage Community Health Officer (CHO) and its teams to undertake successful screenings in their respective regions.
In order to effectively track and monitor the screening program, hospitals must use technology throughout the patient journey.
The use of mHealth and reliable data-gathering software or apps provides ASHAs, ANM, Medical Officer (MO), and experts with information about the patient. A clinical decision support tool for ASHAs can support ASHAs with standard screening criteria and patient triage based on risk level, advocating the best next steps. It can also help to ensure adequate data gathering.
To assist chief medical officers and radiologists across centers in addressing difficulties with workforce capacity, AI-based triaging and imaging solutions can be used. The use of software and IT systems to provide a seamless referral process should be encouraged. This would help in highlighting any dropouts or deviations from the pathway so that the proper actions can be taken.
3. Long Term (> 5 years)
-
(i)
Involvement of community health workers: Government, healthcare system and community participation form the backbone of an effective cancer screening implementation strategy. Local volunteers can augment the breast cancer screening program in low-income settings, and thus, improve outcomes by early diagnosis.120 Community health workers can assist by increasing breast cancer awareness, assisting clinical breast examinations, and navigation of patients through the referral system.
-
(ii)
Addressing financial toxicity due to cancer: A national flagship insurance program -ABPM-JAY, has been functional in India to address certain out-of-pocket expenditure. Globally, it is the largest public health insurance system that covers expenditures upto Rs. 5 Lakhs (6814 USD) per family per year to over 10.74 crores of vulnerable families.103 Further, outpatient financial risk mitigation strategies further need to be designed. digital voucher, E-RUPI,119 launched to cover expensive diagnostic and staging investigations, can also be expanded to pay for cancer medicines.
Furthermore, the Indian government’s efforts to regulate the pricing of anti–HER2 drugs have improved access and outcomes. The launch of low-cost T-DM1 (the antibody-drug conjugate trastuzumab emtansine) and anti–HER2 therapy biosimilars is highly anticipated. The inclusion of newer treatment plans in health benefit packages should be based on empirical evidence (health-technology assessments).
The health information system needs to be linked with other databases including cancer registry, Ayushman Bharat, and death registrations. This will ensure better data capture in terms of cancer registration, follow-up and long-term outcomes.121
-
(iii)
Newer initiatives: Health-tech start-ups are revolutionising different stages of cancer care. For example, Niramai® utilizes machine learning and big data analytics to develop low-cost breast cancer diagnostics.122 Oncostem® assists in personalised treatment decisions using several prognostic biomarkers,123 and UE Lifesciences® uses handheld screening devices that are contactless and radiation-free.124 Panacea® and Mitra Biotech®125 are companies that are developing precision therapies for cancer patients.
There are several patient assistance programs (PAPs) from companies like Roche®, Novartis®, etc., that support patients with cancer in reducing the cost of drugs and navigating patients through their cancer journies. Furthermore, patient navigation strategies are evolving. For example, ‘Kevat’ is the first initiative in India to facilitate patient’s journey from entry to the hospital to subsequent follow-ups during cancer treatment.
In addition, the WHO introduced a Global Breast Cancer Initiative on March 9, 2021, with the goal of reducing global breast mortality by 2.5% by 2040. This initiative aims to reduce 2.5 million global deaths, particularly in low-income countries, where the progress in addressing the disease has been relatively slow. By providing technical support to implement effective strategies for breast cancer management, this evidence-based initiative holds promise for addressing disparities in breast cancer outcomes,126 particularly in low-income countries.
In light of these advancements and initiatives, stakeholders in the field of oncology are encouraged to embrace and support these transformative approaches. Collaborative efforts among health-tech innovators, pharmaceutical companies, healthcare providers, and policymakers are essential for realizing the vision of improved breast cancer care outcomes and reducing the global burden of breast cancer.
Speciality-wise specific gaps and recommendations
Radiodiagnostics
The identified gaps and recommendations to address the gaps in the field of radiodiagnostics are listed in Table 4.
| Observations | Short term recommendations | Long term recommendations |
|---|---|---|
| Low availability of mammography machines | Installation of digital mammography machines in all medical colleges, regional cancer centres and other tertiary care hospitals | Installation of digital mammography machines in all district hospitals |
| Inadequate number of manufacturers of state-of art digital mammography machines in India | Inclusion of digital mammography machines in the list of medical devices with make-in-India exemption to allow global tenders for procurement of machines for government hospitals | Investing in research and development for manufacturing of advanced digital mammography machines. |
| Wide availability of ultrasound machines | Encourage the use of ultrasound for diagnosis of breast diseases in symptomatic women, if mammography is not available | Pooling and analysis of data so generated across hospitals to determine the role of ultrasound in breast cancer diagnosis. |
| Limited availability and expensive nature of MRI and PET-CT facilities | Installation of MRI and PET- CT machines in all medical colleges, regional cancer centres and other tertiary care hospitals | Installation of MRI and PET- CT machines in all district hospitals |
| Optimal utilization of available infrastructure | Education of primary care doctors with recent updates and current concepts directed to prevent under or over use of breast imaging techniques. | Developing clinical guidelines specific to Indian population |
| Wide variations in quality and standard of breast imaging facilities | Defining minimum standards for breast imaging equipment, regular quality assurance tests and practice guidelines which are commensurate with existing infrastructure in the country. These may be optional but actively encouraged at the beginning. Breast Imaging Society of India has already developed and regularly updating such guidelines by expert from across the country. Collaboration with the Society for this purpose may be explored. | Practice guidelines of international standard should to developed. Apex licencing authority may be established for acceptance testing and periodic monitoring of quality of mammography machines, proficiency of manpower and renewal of licences. This may be made a mandatory requirement in future. |
| Training of breast radiographers | Structured mammography training in undergraduate courses of B.Sc. medical technology radiography programs wherever they are running. | Strating post graduate courses of MSc Breast imaging, after B.Sc. in medical technology radiography. |
| Training of radiologists | More emphasis on structured breast imaging and interventions in syllabus of post graduate medical courses of MD (Radio- diagnosis and DNB (Radio- diagnosis). | Recognizing Breast imaging as a distinct superspeciality stream by National Medical Commission and National Board of Examinations |
| Training of radiologists | Increase annual intake in post-doctoral fellowship in breast imaging programs in medical institutes of national importance, where it is already available | Starting of new post- doctoral fellowship in breast imaging programs in all medical institutes of national importance, where it is not already available |
| Continued medical breast education of radiologists and radiographers | Supporting Breast Imaging Society of India for conducting more CMEs and hand on workshops for skill developments in different parts of the country | Regular short term training or observer ships at regional premier centres for radiologists and radiographers working at peripheral government hospitals. |
| Research in breast imaging and interventions | Inviting research proposals and funding the same with specific emphasis on optimal utilization of existing infrastructure, use of AI to address inadequate trained manpower and product developments. | Centralized pooling of breast imaging data form across the country. This may be used to generate country specific data, recommendations and practice guidelines for breast imaging and interventions. |
| Research in screening for breast cancer | Large scale studies on population-based breast cancer screening with clinical breast examination by trained healthcare workers. | Pilot studies on opportunistic and self- volunteered mammographic and/or ultrasound screening in high-risk group women may be conducted to assess its utility. |
MRI: Magnetic resonance imaging, PET-CT: Positron emission tomography - computed tomography, MD: Doctor of medicine, DNB: Diplomate of National Board, CME: Continuing medical education
Pathology
Pathology is a critical specialty involved in the diagnosis of breast cancer. The management of breast cancer is dependent on the identification of subtypes of breast cancer. To address the gaps identified, the following recommendations are suggested.
Careful specimen transport: The specimen should ideally be sent immediately after removal to the histopathology laboratory. In instances where delayed transport is expected, the specimen should be sliced to ensure proper formalin penetration during transport, and this is especially crucial for a mastectomy specimen. Drawing inspiration from the UKNHS, a specimen transport van that carries the specimen in refrigerated boxes should be available at centers that plan to send the specimen out. Additionally, a network of specimen transport systems and sample tracking across the country should be set up at least at far-off places. Make surgeons more aware of the harmful effects of delayed fixation. Training should focus on making controlled single or cruciate incisions into the lesion, preserving the integrity of key margins while allowing for immediate penetration of fixatives.
Audit of biomarkers across each cancer center: European countries have established a system for auditing reported biomarkers in every hospital. This system ensures that any deviations are identified and investigated through root-cause analysis to address issues impacting test results.
Certification for quality pathology services and compulsory participation in an EQAS program: In spite of free NCGEQAS services, most government institutes do not participate to avoid the spotlight. Compulsory EQAS should be complemented with a cancer center’s along with good pathology practice certification.
-
Availability of FDA approved antibodies through a common portal like GEM after cost negotiation.
-
Replacing FISH with D-DISH or validated chromogenic hybridization assays
In most developed nations, a HER2 score of 2+ is reflex tested with FISH. While a molecular laboratory may not be available at most centers, the D-DISH chromogenic in situ hybridization can be performed on the automated Ventana platform, though it requires some training. We have recently published data demonstrating high concordance between D-DISH and FISH. Separate funding will be necessary to support such initiatives.
-
Zonal oncopathology laboratory empowerment with molecular sequencers and know-how to generate patient population-specific panels.
-
Development of gene-based predictive test akin to OncotypeDx in Indian population.
Surgery
To address critical gaps and deficiencies in human resources for breast cancer treatment in India, it’s essential to implement targeted strategies across different timeframes. Here are recommendations classified based on their implementation timeframe.
1. Immediate (1-2 years)
(i) Training programs: Establish short-term training programs and workshops for healthcare professionals, including oncologists, surgeons, radiologists, pathologists, nurses, and allied health workers, to enhance their knowledge and skills in breast cancer diagnosis, treatment, and survivorship care.
(ii) Capacity building: Provide intensive training and certification courses for primary care physicians and nurses to improve their competency in clinical breast examination, early detection, and referral of breast cancer cases.
(iii) Task shifting: Explore the feasibility of task shifting by training allied health workers, such as community health workers and mid-level providers, to perform basic breast cancer screening, patient education, and supportive care services.
2. Medium-term (3-5 years)
(i) Specialized fellowships: Expand fellowship programs in breast oncology, surgical oncology, medical oncology, radiation oncology, and breast imaging to train a cadre of specialized healthcare professionals dedicated to breast cancer care.
(ii) Interdisciplinary training: Develop interdisciplinary training programs and tumor boards involving oncologists, surgeons, radiologists, pathologists, nurses, and psychologists to foster collaboration and improve care coordination for breast cancer patients.
(iii) Workforce redistribution: Implement policies to incentivize healthcare professionals to work in underserved areas by offering financial incentives, career development opportunities, and infrastructure support.
3. Long-term (>5 years)
(i) Academic Partnerships: Foster collaborations between medical institutions, academic universities, and research centers to establish comprehensive breast cancer training and research programs, including postgraduate courses and research fellowships.
(ii) Subspecialty certification: Work towards establishing subspecialty certification in breast oncology for healthcare professionals to recognize expertise and promote standardized care delivery in breast cancer treatment.
(iii) Continuing education: Develop continuous professional development programs, online courses, and tele-education platforms to facilitate ongoing learning and skill enhancement for healthcare professionals engaged in breast cancer care.
(iv) Health workforce planning: Conduct workforce needs assessments and long-term planning to anticipate future demand for breast cancer care services and ensure adequate human resources capacity to meet growing needs.
Collaboration among government agencies, professional associations, academic institutions, and healthcare providers is essential for the successful implementation of these programs.
Medical oncology
Access to drugs: With the evolution of systemic therapies for breast cancer, the cost of treatment has increased significantly. Access to newer therapies, when introduced in the Indian market, is extremely limited during the patent period; for example, when trastuzumab was introduced, less than 10% of patients in India could integrate it into their treatment despite significant benefits.127 However, with the availability of Indian generic versions, usage increased to over 50% Additionally, integration into the PM-JAY program is expected to enable over 80% of patients to access trastuzumab in their treatment.128 A shorter duration of trastuzumab has been advocated as a more effective strategy in India.129 Similarly, there are several drugs, including but not limited to CDK4/6 inhibitors, immunotherapy (pembrolizumab), and newer anti-HER2 agents, that remain inaccessible to most patients.
Therefore, drugs used in the management of breast cancer have been categorized into essential and optimal classifications for non-metastatic and metastatic scenarios, respectively [Table 5]. The essential classification includes drugs that demonstrate significant efficacy in terms of disease-free survival or overall survival through randomized controlled trials and are currently accessible in India as of March 2024. Conversely, drugs are designated as optimal if there exists definitive evidence of enhanced disease-free survival, notwithstanding the absence of overall survival data, or if the drugs remain largely inaccessible due to prohibitively high costs. Thus, access to all essential drugs is imperative for all patients diagnosed with breast cancer, with the additional aim of providing access to optimal drugs. Given the evolving landscape of evidence, periodic reevaluation and potential reclassification of drugs may be warranted in the future. Furthermore, ongoing clinical trials investigating novel drugs may lead to their integration into breast cancer treatment protocols, necessitating regular updates to the drug classification list.
| Early and locally advanced | Metastatic | |
|---|---|---|
| Essential drugs |
Doxorubicin Epirubicin Cyclophosphamide Paclitaxel Docetaxel Carboplatin Capecitabine 5-Fluorouracil Methotrexate Trastuzumab (A) Trastuzumab emtansine (A) (TDM-1) Leuprolide Goserelin Tamoxifen Anastrazole Letrozole Exemestane Olaparib (A) Zoledronate Denosumab |
Doxorubicin Epirubicin Cyclophosphamide Paclitaxel Docetaxel Gemcitabine Nab-paclitaxel Carboplatin Capecitabine 5-Fluorouracil Methotrexate Trastuzumab Trastuzumab emtansine (4) (TDM-1) Tamoxifen Leuprolide Goserelin Anastrazole Letrozole Exemestane Zoledronate Denosumab Olaparib (4) Talazoparib (4) Vinorelbine Eribulin (2) Palbociclib (3/4) Ribociclib (4/5) Abemaciclib (3/4) Fulvestrant (2) Pertuzumab (4) Lapatinib (3/4) Everolimus (2) |
| Optimal |
Pembrolizumab (A) Pertuzumab (A) Abemaciclib (A) Ribociclib |
Trastuzumab deruxtecan (T- DXd) (4) Tucatinib (4) Sacituzumab govitecan (4) Alpelisib (2) Pembrolizumab (4) |
| Currently not available in India |
Elacestrant (3) Capivasertib (3) |
The list also includes the European Society of Medical Oncology (ESMO) magnitude of clinical benefit scale (MCBS), which is designed to enhance decision-making concerning the value of anti-cancer treatments. Its aim is to ensure fair access and reduce disparities in cancer care.130 The MCBS helps oncologists communicate treatment benefits to patients and assists health policymakers in prioritizing therapies for reimbursement. It is currently integrated into ESMO Clinical Practice Guidelines and utilized in Health Technology Assessment processes globally.
Briefly, the ESMO-MCBS grading system delineates treatments that substantially enhance patient survival or quality of life from those with more limited benefits based on outcomes from randomized clinical trials.131,132 This evaluation encompasses factors, including overall survival, progression-free survival, and quality of life, providing a comprehensive framework for assessing cancer medicines. Therapies that achieve higher ESMO-MCBS scores, particularly those falling into categories A and B for curative intent and scores 4 and 5 for non-curative setting, warrant expedited evaluation for value and cost-effectiveness.
Access to genetic testing: Approximately 18% of individuals diagnosed with breast cancer may have a pathogenic or likely pathogenic mutation within one of the genes associated with hereditary breast cancer predisposition.132 Such genetic anomalies carry significant clinical ramifications, including the implementation of risk reduction strategies for the affected individual, such as intensified surveillance protocols or prophylactic surgeries. Additionally, these mutations may influence therapeutic decisions, with the emergence of PARP inhibitors such as olaparib as potential treatment options. Furthermore, there is a cascade effect necessitating genetic testing for at-risk family members. In the Indian context, a consensus document has been previously published outlining guidelines for genetic counseling, testing, and management of hereditary breast and ovarian cancer.133 Therefore, ensuring access to germline testing for eligible breast cancer patients is paramount. Such testing should include a comprehensive multigene panel analysis using next-generation sequencing techniques, supplemented by reflex testing using multiplex ligation-dependent probe amplification assays (MLPA) to detect large genomic rearrangements.
Essential recommendations:
Access to a genetic counsellor
Access to germline testing with a multigene panel and reflex
Human resources for delivering systemic treatment: The systemic treatment of breast cancer has evolved significantly over the last decades. It is arguably the most complex part of treatment, where a trained medical oncologist is required to provide an optimal systemic treatment strategy. Moreover, systemic treatments are associated with unique side effects that require trained and experienced personnel to identify and treat such treatment-related complications. In a recent survey in 2022, it was estimated that India needs approximately 5,000 medical oncologists to address the rising burden of cancer, while there are only 2000 specialists available in the country.134 This leads to a higher clinical burden on the existing Medical Oncologists in India, as well as poor access of patients with breast cancer to comprehensive multidisciplinary care.97
Therefore, we recommend that all medical colleges in India must have a department of Medical Oncology to ensure optimal management of patients with breast cancer. This department should ideally be staffed with trained medical oncologists who possess qualifications such as DM or DNB in medical oncology. However, in cases where a trained medical oncologist is not available, the responsibility for managing patients with breast cancer can temporarily fall under the Department of Internal Medicine.
Given that there are 706 National Medical Commission (NMC)-recognized Medical Colleges in India as of March 2024, each of these institutions must meet the requirement of having a Department of Medical Oncology or a suitable alternative arrangement in place for managing breast cancer patients. This requirement ensures that patients receive appropriate care and treatment for their condition.
Radiation oncology
Key issues/gaps in the human resource component
(i) Immediate: There is an urgent need to ensure an appropriate understanding of hypofractionated radiotherapy practices that are emerging in breast cancer. Clinicians and Medical Physicists must attend breast cancer site-specific workshops to understand the nuances of breast radiotherapy planning. National and International bodies should be approached immediately to ensure multiple courses on breast radiotherapy planning are available through various regional centers across the country.
It is imperative that, alongside the enhancement of infrastructure for radiotherapy across India, the shortage of skilled manpower in radiotherapy practices is addressed. Nodal centers must be encouraged to start training programs on Medical Physics and Radiotherapy technician courses.
A nationwide consortium is essential to ensure that patient-specific quality assurance practices are maintained across all treating centers. A national breast cancer radiotherapy treatment protocol is essential. This should encompass all aspects of breast radiotherapy treatment, be practical, and should address all issues needed for treatment in cobalt and linear accelerator-based centers. This protocol can be implemented in a phased manner, starting with regional centers agreeing on the processes, followed by smaller centers across the country.
Government and non-government institutions must be encouraged to allow external peer review of breast radiotherapy services and protocols.
Additionally, it must be essential for all clinical staff to attend communication skills programs across the country. This could include mandatory attendance of workshops and certification courses. The government must ensure repeated revision courses with a gap of 3-5 years to allow continued education.
Radiotherapy technicians must undergo image guidance training and should be certified for the same. Programs must be available nationally to do so.
Under-utilization of palliative breast radiotherapy for regional symptom control and improving patient quality of life remains a matter of concern. Various reasons, including referral bias and lack of coherent multidisciplinary team working, could be contributory factors. Enhancing knowledge and breast cancer, multidisciplinary team working is an area of need, and national policies to enroll multidisciplinary team (MDT) across the country and provide guidance for successful protocol-driven teamwork should be encouraged.
(ii) Medium-term: The Government of India plans to open one medical college in each district across the country. Radiotherapy departments must be made essential, at least within two adjacent districts, within the next five years.
New centers for training Medical Physicists and Radiotherapy technicians should be commissioned across the country.
A bridging course should be allowed to train radiotherapy technicians to allow them to plan radiotherapy treatments under supervision (dosimetrists). This will allow the technicians to have a career pathway and also help institutions safely address radiotherapy planning bottlenecks. The government should work with nodal bodies such as the Atomic Energy Regulatory Board to establish such courses.
Radiotherapy-specific nurses must be trained across the country to allow appropriate management of any and all adverse events and provide patients with information and support during therapy. ASHA workers should also be trained to counsel patients on their breast radiotherapy treatment and highlight any unaddressed adverse events.
(iii) Long term: Each center must have site-specific (breast cancer) radiation oncologists and radiotherapy planners.
Nodal centers should be identified, and leaders in the field must be encouraged to draw a breast cancer radiotherapy-specific audit and research plan.
(iv) Key issues/gaps in the current infrastructure, facilities, technologies, policies, programs, etc.: More centers that allow patients to get radiotherapy treatment near their homes must be encouraged. Such centers must have essential quality assurance systems, SOPs, and audit processes in place.
All centers treating breast cancer must be encouraged to provide cardiac-sparing radiotherapy strategies, at least using multi-leaf collimators. Given the strong relationship between heart dose and cardiac morbidity and mortality, it is important that hardware (multi-leaf collimators, gating cameras) and compatible software are available in all centers.
All centers, including smaller centers, should be included within a national program to establish clinical protocol-based treatment processes in breast radiotherapy. This would include internal audit processes.
All centers should participate in an external peer review process.
A national audit process must be in place that will allow analysis, collation, and publication of treatment-related near misses and accidents.
The audit process above should also ensure that site-specific outcomes of breast cancer radiotherapy planning are in place.
Recommendations
(i) Immediate: Create a state-wise map of available RT facilities and catchment areas to identify underserved regions.
Develop a national detailed radiotherapy planning SOP.
Develop a detailed quality assurance SOP for treatment planning and delivery. The SOP should include mandatory and optimal recommendations. It is preferred that a group of competent experts develop the same.
(ii) Medium and long term: Cancer therapy/radiation Oncology department may be made mandatory criteria for recognition of medical colleges. The departments can be set up with one Professor, one Associate Professor, and, one Assistant Professor, and senior residents for one unit (as mandated by NMC) initially to start the service run outpatient (OPs) and inpatient (IP) service. Radiotherapy machine (at least one linac) installation is to be performed within the next 3 years. Two medical physicists and four radiotherapy technicians should be the minimum number of paramedical staff members to run a linear accelerator.135
Identify a District/General/Taluk hospital where an RT machine could be installed to work on the hub and spoke (satellite radiotherapy center) model with a cancer center/medical college. Patients could travel to the hub center for simulation and RT planning (1-2 visits) and receive treatment at a peripheral unit closer to home. There should be internet connectivity between the centers for online transmission of RT plans. The peripheral unit could work on a single-shift basis with a minimum staff requirement as required by AERB. The peripheral unit could be under the administrative control of the parent center with regard to the RT program. (3-5 years and beyond five years, depending on the needs of the locality).
Identify major cancer centers in each state that can serve as facilitators - providing guidance on quality assurance standards and conducting QA audits. This initiative can be structured over different timeframes: 1-2 years for initial identification and planning 3-5 years for implementation and training, and beyond 5 years for ongoing support and assessment, depending on the availability of human resources and infrastructure.
(iii) Key issues/gaps in the current funding scenario in the country: Accessibility of radiotherapy can be improved with a plan for delivering radiotherapy near home, using the above-mentioned hub-spoke model. Infrastructure funding for the same is necessary.
Funding for enhancing quality in breast cancer therapy is required for appropriate national group functioning to deliver SOP, audit, and peer review, which is of utmost need.
Clinical trials specifically related to testing radiotherapy techniques and hypotheses in India must be prioritized. Radiotherapy, being one of the most cost-effective measures, allows such infrastructure funding to reap quick benefits with a reduction in patient care costs.
Training programs and workshops for doctors (radiation oncologists) on-site specializing in the safe use of modern techniques such as regional nodal radiotherapy, ultra hypofractionation, etc., should be funded. The medical physics team is to be upskilled for the same purposes.
A national audit database on core outcome parameters must be commissioned. This funded body must ensure the quality of uploaded data and should be allowed to peer review centers. Such audit should link to international endeavors on service enhancement and further enhance the quality of cancer care,
A program for developing training for communication skills is essential, and funding for workshops for the same is likely to directly improve patient care.
Workshops for training specialist breast care nurses and ASHA workers will need funding and support.
Recommendations
(i) Immediate: Seed funding from government agencies to initiate high-quality multicentric audits across Indian centers will soon affect the quality of care. This funding will promote transparency, and a peer review process will improve the standards of breast radiotherapy care. Such audits will facilitate the establishment of a national database of key outcomes, identify areas of need, enhance protocol-based working patterns, and highlight areas requiring research.
Bridging courses for competent radiation technicians to function as dosimetrists under the supervision of medical physics staff could be funded through the national skill development program. This will ease planning bottlenecks.
Certificate programs should be encouraged for all staff to upskill their breast radiotherapy practices.
Communication skills training programs should be made mandatory, and funded workshops should be encouraged.
(ii) Medium and long term: Setting up an RT facility requires intense capital outlay for the purchase of equipment, construction to conform to radiation safety standards, quality assurance, and personnel. Although the initial investment in setting up RT machines is high, modern linacs are versatile and run for 12-15 years without major breakdowns.
Encourage public-private participation in setting up RT facilities. (3-5 years).
Explore collaboration with organizations such as IAEA for funding for the RT unit. (3-5 years). The IAEA Rays of Hope initiative (RoH) is aimed at assisting member states in establishing/expanding capacities in radiotherapy and multimodality medical imaging. The initiative will support the designated RoH Anchor Centers, thereby strengthening and expanding their capacity to conduct critical work more effectively. RoH emphasizes equipment, training, research/innovation, and support for high-impact interventions for cancer patients, thereby contributing directly to the achievement of the United Nations Sustainable Development Goal.136
Collaboration with RT machine vendors for a bulk order of work-horse linacs with the capability of 3DCRT and IMRT with KV (2D) imaging for installation at multiple sites so that total cost may be reduced. (3-5 years)
Government subsidy or relaxation in statutory taxes for the infrastructure investment by the government/government autonomous and not-for-profit organizations must be encouraged. ((>5 years)
Explore the possibility of having the international vendors set up their manufacturing units in India. (>3 years)
The manufacture of high-quality indigenous linear accelerators is essential. These machines should deliver the same precision as imported models, be user-friendly, and provide timely, high-quality service. This initiative should be targeted for development over a timeframe of more than five years.
Patients can receive treatment through government insurance schemes. However, the loss of wages for the caregiver and other costs are a significant hurdle. Providing sustenance amounts for caregivers during the period of radiotherapy to compensate for the loss of wages (only for caregivers who are unemployed/not more than unskilled laborers and conditional on the patient completing the planned treatment). This support could serve as an incentive to complete treatment.
Key issues/gaps in the current policymaking.
(i) Immediate: Ensure a strong steering group is formed that drives a national breast cancer radiotherapy delivery program. This should include developing an SOP by consensus, driving QA processes, and developing an audit program.
Mandate setting up of Cancer Therapy/RO dept in all medical colleges - NMC notification
Consider subsidizing/incentivizing centers investing in cancer infrastructure in areas of need (at least for Govt & Govt grant-in-aid institutions).
Commission a multidisciplinary group that enhances breast cancer research, which examines India-specific solutions on breast cancer radiotherapy areas of need.
Strategize to work with regulatory authorities to provide bridging programs for radiotherapy technicians and nurses.
(ii) Medium and long term: Develop Private-Public relationships and commission more cancer centers delivering radiation therapy near patients’ homes.
Work with regulatory bodies to train dosimetrists.
Incentivize training of medical physicists and radiation therapy technicians.
Key issues and gaps in research infrastructure, human resources, and output. Recommendations to bridge the research gaps
(i) Immediate: Develop customized bridging courses and certificate programs for enhancing breast radiotherapy skills for doctors, medical physicists, dosimetrists, and radiotherapy technologists.
Fund a national outcome audits database that documents and reviews key radiotherapy outcomes in breast cancer.
Funding for clinical research addressing efficacy and safety issues in RT is relevant to India and is expected to benefit most patients.
WAY FORWARD
Suggested policy activities and advocacy for policymakers
Equitable distribution of cancer care services
Inequitable distribution of cancer care services has been identified as one of the major barriers to providing comprehensive breast cancer care in the country. Focusing on underserved areas in the development of cancer care infrastructure can help overcome this barrier.
Manpower training
There is a significant shortage of human resources especially medical oncologists and medical physicists. With the development of infrastructure, trained manpower will be required for treating cancer patients. Many medical colleges associated with tertiary care centers currently lack these departments. Policymakers may consider establishing these departments in top medical colleges where adequate training can be provided in the future to overcome this barrier.
Expansion of infrastructure
In diagnostics, there is a critical need for digital mammography machines and radiotherapy services to treat patients with breast cancer in India.
Improved access to systemic treatment drugs
The cost of breast cancer treatment is increasing as newer and more effective drugs are incorporated into treatment. We have classified the drugs as essential and optimal [Table 5]. Access to essential drugs should be improved to increase the proportion of patients who can receive such treatment.
Access to genetic counseling and testing
Approximately 18% of women with breast cancer have a genetic mutation. Despite a significant decrease in the cost of testing, access to genetic testing remains limited for many patients. The inclusion of genetic counselor teleconsultation and genetic testing in PM-JAY may improve access for a significant number of beneficiaries.
Screening program for breast cancer
The incidence of breast cancer in urban India is steadily increasing. We need a two-pronged approach. A screening program with clinical breast examination and an augmentation of diagnostic mammography infrastructure may be cost-effective for the Indian scenario.
Awareness programs
A significant proportion of patients present with advanced breast cancer. Programs using mass media channels have the potential to increase public awareness of breast cancer.
Industry-academia partnership for research and development
Most innovation in breast cancer treatment originates from the West, resulting in the ever-increasing cost of newer cancer drugs. Industry-academia partnerships and funding for research and development are needed to bring innovation from the countryside and keep the cost of cancer care under control.
Recommendations for health/medical professionals
Early introduction of breast cancer in curriculum of medical education:
Every physician in the country should be familiar with common cancer presentations and diagnostic workups. There is a significant delay between the presentation of a patient and the appropriate referral to an oncology center. This can be reduced by including breast cancer early in medical education.
Training programs
Establish short-term educational programs and workshops for healthcare professionals, including oncologists, surgeons, radiologists, pathologists, nurses, and allied health professionals, to improve their knowledge and skills in breast cancer diagnosis, treatment, and survivorship care.
Interdisciplinary training
Develop interdisciplinary training programs and tumor boards involving oncologists, surgeons, radiologists, pathologists, nurses, and psychologists to foster collaboration and improve care coordination for breast cancer patients.
Suggestions to create awareness among the general public, NGOs, and community stakeholders
Patient advocacy
There is a significant unmet need for breast cancer patient advocates in India who can play a critical role in bridging the gap between policy makers and patients. NGOs and community stakeholders need to form such disease- specific patient advocacy groups.
Awareness campaigns
In addition to policy makers, NGOs and community stakeholders need to be involved in awareness campaigns. Key community stakeholders can be selected as ambassadors for such campaigns.
Philanthropic donations for access to treatment and research
Most research funding in India comes from government agencies. However, NGOs can play a critical role in channeling philanthropic donations to improve access to treatment and promote indigenous research.
Patient support groups
Patient support groups can help patients navigate their treatment and survivorship issues, and NGOs can help bring together patients and survivors from multiple oncology centers to address their needs..
ACKNOWLEDGMENT
We acknowledge the contributions of Dr. Palak Popat (TMH, Mumbai), Dr. Anitha Sen (RCC, Trivandrum), and Dr. Nameeta Mohindra (SGPGI, Lucknow) for providing valuable information in the radiodiagnosis section.
References
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
- [CrossRef] [PubMed] [Google Scholar]
- Three-year report of the population based cancer registries 2012–2014. Bengaluru: National Cancer Registry Programme, Indian Council of Medical Research (ICMR); 2017.
- GLOBOCAN 2020 Report on global cancer burden: challenges and opportunities for surgical oncologists. Ann Surg Oncol. 2022;29:6497-500.
- [CrossRef] [PubMed] [Google Scholar]
- Planning for tomorrow: Global cancer incidence and the role of prevention 2020-2070. Nat Rev Clin Oncol. 2021;18:663-72.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;2021
- [CrossRef] [Google Scholar]
- Risk factors for the development of triple-negative breast cancer versus non-triple-negative breast cancer: A case-control study. Sci Rep. 2023;13:13551.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of screening by clinical breast examination on breast cancer incidence and mortality after 20 years: Prospective, cluster randomised controlled trial in Mumbai. BMJ. 2021;372:n256.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Improving accuracy of breast cancer biomarker testing in India. Indian J Med Res. 2017;146:449-58.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A clinicopathological analysis of molecular subtypes of breast cancer using immunohistochemical surrogates: A 6-year institutional experience from a tertiary cancer center in North India. South Asian J Cancer. 2023;12:104-11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinicopathological profile of breast cancer: An institutional experience. Indian J Cancer. 2018;55:210-3.
- [CrossRef] [PubMed] [Google Scholar]
- External quality assurance helps improve infrastructure for testing breast biomarkers across a lower- and middle-income country: ur experience with breast biomarker testing in the national cancer grid external quality assurance system in India. Arch Pathol Lab Med. 2024;148:1028-34.
- [CrossRef] [PubMed] [Google Scholar]
- Ki67--no evidence for its use in node-positive breast cancer. Nat Rev Clin Oncol. 2015;12:296-301.
- [CrossRef] [PubMed] [Google Scholar]
- Ki-67 in breast cancer: Simulacra and simulation. Indian J Cancer. 2020;57:231-233.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus guidelines for the management of HR-positive HER2/neu negative early breast cancer in India, SAARC region and other LMIC by DELPHI survey method. BMC Cancer. 2023;23:714.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The usefulness of CanAssist breast in the assessment of recurrence risk in patients of ethnic Indian origin. Breast. 2021;59:1-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Breast cancer in India: Present scenario and the challenges ahead. World J Clin Oncol. 2022;13:209-218.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinico-epidemiological profile of breast cancer patients and the retrospective application of Gail model 2: An Indian perspective. Breast Dis. 2016;36:15-22.
- [CrossRef] [PubMed] [Google Scholar]
- Breast cancer survival in India across 11 geographic areas under the National Cancer Registry Programme. Cancer. 2024;130:1816-25.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707-16.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127-35.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Radiotherapy to regional nodes in early breast cancer: An individual patient data meta-analysis of 14 324 women in 16 trials. Lancet. 2023;402:1991-2003.
- [CrossRef] [PubMed] [Google Scholar]
- Adjuvant radiation therapy in breast cancer: Recent advances & Indian data. Indian J Med Res. 2021;154:189-98.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Alleviating morbidity from locally advanced breast cancer using a practical and short radiation therapy regimen: results of the HYPORT palliative studies. Int J Radiat Oncol Biol Phys. 2023;116:1033-42.
- [CrossRef] [PubMed] [Google Scholar]
- Government of India. Ayushman Bharat. Comprehensive Primary Healthcare through Health and Wellness Centres. Operational Guidelines; Available from: https://www.nhm.gov.in/New_Updates_2018/NHM_Components/Health_System_Stregthening/Comprehensive_primary_health_care/letter/Operational_Guidelines_For_CPHC. [Last accessed 2024 Sep 03].
- Government of India. Press information bureau. New Delhi: Ministry of Health & Family Welfare Initiatives & Achievements-2023; 2023. Available from: https://pib.gov.in/PressReleasePage.aspx?PRID=1990674#:∼:text=Similarly%2C%20these. [Last accessed 2024 Sep 03]
- Government of India. Press Information Bureau. Update on cancer care plan and management. 2023. Available from: https://pib.gov.in/Pressreleaseshare.aspx?PRID=1911468. [Last accessed 2024 Sep 03]
- Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: A population-based study. Lancet Oncol. 2019;20:769-80.
- [CrossRef] [PubMed] [Google Scholar]
- Expanding global access to radiotherapy. Lancet Oncol. 2015;16:1153-86.
- [CrossRef] [PubMed] [Google Scholar]
- Delivery of affordable and equitable cancer care in India. Lancet Oncol. 2014;15:e223-33.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: https://pib.gov.in/PressReleasePage.aspx?PRID=1897984. [Last accessed 2024 Sep 03].
- Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432-44.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Anthracycline-containing and taxane-containing chemotherapy for early-stage operable breast cancer: A patient-level meta-analysis of 100 000 women from 86 randomised trials. Lancet. 2023;401:1277-92.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Moment of truth-adding carboplatin to neoadjuvant/adjuvant chemotherapy in triple negative breast cancer improves overall survival: An individual participant data and trial-level Meta-analysis. Breast. 2022;64:7-18.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Addition of platinum to sequential taxane- anthracycline neoadjuvant chemotherapy in patients with triple-negative breast cancer: A phase III randomized controlled trial. 2022 San Antonio Breast Cancer Symposium. Abstract GS5-01. Presented December 9, 2022.
- Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147-59.
- [CrossRef] [PubMed] [Google Scholar]
- Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771-84.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805-16.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341-52.
- [CrossRef] [PubMed] [Google Scholar]
- Adjuvant anastrozole versus exemestane versus letrozole, upfront or after 2 years of tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): A randomised, phase 3 trial. Lancet Oncol. 2018;19:474-85.
- [CrossRef] [PubMed] [Google Scholar]
- Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27--a randomized controlled phase III trial. J Clin Oncol. 2013;31:1398-404.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparative efficacy and safety of adjuvant letrozole versus anastrozole in postmenopausal patients with hormone receptor-positive, node-positive early breast cancer: Final results of the randomized phase iii femara versus anastrozole clinical evaluation (FACE) trial. J Clin Oncol. 2017;35:1041-48.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of ovarian ablation or suppression on breast cancer recurrence and survival: Patient-level meta- analysis of 14,993 pre-menopausal women in 25 randomized trials. J Clin Oncol. 2023;41:503.
- [Google Scholar]
- Therapeutic bone-modifying agents in the nonmetastatic breast cancer setting: The controversy and a value assessment. Am Soc Clin Oncol Educ Book. 2017;37:116-22.
- [CrossRef] [PubMed] [Google Scholar]
- Role of bone-modifying agents in metastatic breast cancer: An American society of clinical oncology-cancer care ontario focused guideline update. J Clin Oncol. 2017;35:3978-86.
- [CrossRef] [PubMed] [Google Scholar]
- Trastuzumab for early-stage, HER2-positive breast cancer: A meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021;22:1139-50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evaluation of 1-year vs shorter durations of adjuvant trastuzumab among patients with early breast cancer: An individual participant data and trial-level meta-analysis. JAMA Netw Open. 2020;3:e2011777.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Adjuvant Pertuzumab and Trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J Clin Oncol. 2021;39:1448-57.
- [CrossRef] [PubMed] [Google Scholar]
- Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617-28.
- [CrossRef] [PubMed] [Google Scholar]
- Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401:105-17.
- [CrossRef] [PubMed] [Google Scholar]
- Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783-91.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384:2394-405.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol. 2022;33:1250-68.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523-33.
- [CrossRef] [PubMed] [Google Scholar]
- Adjuvant abemaciclib plus endocrine therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative, high-risk early breast cancer: Results from a preplanned monarche overall survival interim analysis, including 5-year efficacy outcomes. J Clin Oncol. 2024;42:987-93.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Adjuvant palbociclib for early breast cancer: The PALLAS trial results (ABCSG-42/AFT-05/BIG-14-03) J Clin Oncol. 2022;40:282-93.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer-The penelope-B trial. J Clin Oncol. 2021;39:1518-30.
- [CrossRef] [PubMed] [Google Scholar]
- Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2- early breast cancer: Primary results from the phase III NATALEE trial. J Clin Oncol.. 2023;41:LBA500.
- [Google Scholar]
- MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638-46.
- [CrossRef] [PubMed] [Google Scholar]
- Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925-36.
- [CrossRef] [PubMed] [Google Scholar]
- Overall Survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386:942-50.
- [CrossRef] [PubMed] [Google Scholar]
- Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556-67.
- [CrossRef] [PubMed] [Google Scholar]
- Metronomic chemotherapy for metastatic breast cancer. Oncol Res Treat. 2022;45:12-17.
- [CrossRef] [PubMed] [Google Scholar]
- Indicator metadata registry details. Available from: https://www.who.int/data/gho/indicator-metadata-registry/imr-details. [Last accessed 2024 Sep 03].
- Available from: https://www.unfpa.org/data/world-population/IN. [Last accessed 2024 Sep 03].
- Available from: https://www.aerb.gov.in/images/PDF/Radiotheraphy/RSD3.pdf. [Last accessed 2024 Sep 03].
- The impact of scaling up access to treatment and imaging modalities on global disparities in breast cancer survival: A simulation-based analysis. Lancet Oncol. 2021;22:1301-11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ten-year results of FAST: A randomized controlled trial of 5-fraction whole-breast radiotherapy for early breast cancer. J Clin Oncol. 2020;38:3261-72.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hypofractionated radiation therapy comparing a standard radiotherapy schedule (over 3 weeks) with a novel 1-week schedule in adjuvant breast cancer: An open-label randomized controlled study (HYPORT-Adjuvant)-study protocol for a multicentre, randomized phase III trial. Trials. 2020;21:819.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Access to radiation therapy: From local to global and equality to equity. JCO Glob Oncol. 2022;8:e2100358.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Human resources for cancer control in uttar pradesh, India: A case study for low and middle income countries. Front Oncol. 2014;4:237.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Planning National Radiotherapy Services: A Practical Tool 2011. Available from: https://www-pub.iaea.org/books/IAEABooks/8419/Planning-National- Radiotherapy-Services-A-Practical-Tool. [Last accessed 2024 Sep 03].
- Strengthening of cancer care in India. Available from: https://pib.gov.in/Pressreleaseshare.aspx?PRID=1546948. [Last accessed 2024 Sep 24].
- Patient navigation pathway and barriers to treatment seeking in cancer in India: A qualitative inquiry. Cancer Epidemiol. 2013;37:973-8.
- [CrossRef] [PubMed] [Google Scholar]
- The Andersen model of total patient delay: A systematic review of its application in cancer diagnosis. J Health Serv Res Policy. 2012;17:110-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Patient-mediated factors predicting early- and late-stage presentation of breast cancer in Egypt. Psychooncology. 2011;20:532-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence and determinants of late-stage presentation among cervical cancer patients, a systematic review and meta-analysis. PLoS One. 2022;17:e0267571.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31:58-69.
- [CrossRef] [PubMed] [Google Scholar]
- Perceived income adequacy among older adults in 12 countries: Findings from the survey of health, ageing, and retirement in Europe. Gerontologist. 2009;49:397-406.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Socio-economic and health access determinants of breast and cervical cancer screening in low-income countries: Analysis of the world health survey. PLoS One. 2012;7:e48834.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Do socio-demographic factors influence mammography use of French women? Analysis of a French cross-sectional survey. Eur J Cancer Prev. 2006;15:219-24.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of the impact of stigma and nihilism on lung cancer outcomes. BMC Cancer. 2012;12:184.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The psychosocial impact of stigma in people with head and neck or lung cancer. Psychooncology. 2013;22:140-52.
- [CrossRef] [PubMed] [Google Scholar]
- Lung cancer stigma, anxiety, depression, and quality of life. J Psychosoc Oncol. 2014;32:59-73.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Perceived stigma, self-blame, and adjustment among lung, breast and prostate cancer patients. Psychol Health. 2009;24:949-64.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer-Related Stigma and Depression in Cancer Patients in A Middle-Income Country. Asia-Pac J Oncol Nurs.. 2019;7:95-102.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Meta-synthesis exploring barriers to health seeking behaviour among Malaysian breast cancer patients. Asian Pac J Cancer Prev. 2015;16:145-52.
- [CrossRef] [PubMed] [Google Scholar]
- Use of alternative medicine is delaying health-seeking behavior by bangladeshi breast cancer patients. Eur J Breast Health. 2018;14:166-72.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Use of complementary and alternative medicine among patients with cancer in a sub-Himalayan state in India: An exploratory study. J Ayurveda Integr Med. 2021;12:126-30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Journeys: Understanding access, affordability and disruptions to cancer care in India. Ecancermedicalscience. 2022;16:1342.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A study on awareness on ab-pmjay for treatment of diseases with special reference to cancer care in Thanjavur district of Tamil Nadu.
- Study on awareness and utilization of Ayushman bharat arogya karnataka scheme in Chamarajanagar taluk: a cross-sectional study. Int J Res Med Sci. 2023;11:544-50.
- [Google Scholar]
- FICCI and EY. Call for action: Making quality cancer care more accessible and affordable in India. Available from: https://assets.ey.com/content/dam/ey-sites/eycom/en_in/topics/health/2022/ey-making-quality-cancer-care-more-accessible-andaffordable-in-india.pdf. [Last accessed 2024 Sep 03].
- Barriers to cervical cancer and breast cancer screening uptake in low- and middle-income countries: A systematic review. Health Policy Plan. 2023;38:509-27.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Health system barriers influencing timely breast cancer diagnosis and treatment among women in low and middle-income Asian countries: Evidence from a mixed-methods systematic review. BMC Health Serv Res. 2022;22:1601.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Global survey of clinical oncology workforce. J Glob Oncol. 2018;4:1-12.
- [CrossRef] [Google Scholar]
- Projected cancer burden, challenges, and barriers to cancer prevention and control activities in the state of Telangana. PLoS One. 2023;18:e0278357.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Medical oncology in India: Workload, infrastructure, and delivery of care. Indian J Med Paediatr Oncol. 2019;40:121-7.
- [CrossRef] [Google Scholar]
- Booklet on Universal Health Coverage (UHC).
- Knowledge of risk factors and early detection methods and practices towards breast cancer among nurses in Indira Gandhi Medical College, Shimla, Himachal Pradesh, India. Asian Pac J Cancer Prev. 2013;14:117-20.
- [CrossRef] [PubMed] [Google Scholar]
- Time interval between self-detection of symptoms to treatment of breast cancer. Asian Pac J Cancer Prev. 2020;21:169-74.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The dynamics of breast cancer screening approaches in urban India: An ethnographic study from Delhi. SSM - Qual Res Health. 2022;2:100135.
- [CrossRef] [Google Scholar]
- NHA | Setu Dashbaord [Internet]. Available from: https://dashboard.pmjay.gov.in/pmj/#/. [Last accessed 2024 Apr 12].
- Financial toxicity of cancer treatment in India: Towards closing the cancer care gap. Front Public Health. 2023;11:1065737.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comprehensive value-based cancer care in India: Opportunities for systems strengthening. Indian J Med Res. 2021;154:329-37.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Population-based cancer screening programmes in low-income and middle-income countries: Regional consultation of the International Cancer Screening Network in India. Lancet Oncol. 2018;19:e113-e122.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Strengthening breast cancer screening program through health education of women and capacity building of primary healthcare providers. Front Public Health. 2023;11:1276853.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Model for early detection of breast cancer in low-resource areas: The experience in Peru. J Glob Oncol. 2018;4:1-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Indian Cancer Patients’ use of Traditional, Complementary and Alternative Medicine (TCAM) and delays in presentation to Hospital. Oman Med J. 2009;24:99-102.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of the use of traditional complementary and alternative medicine amongst cancer patients in a tertiary care center in Kerala, India. J Ayurveda Integr Med. 2021;12:359-64.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Expanding the horizon for breast cancer screening in India through artificial intelligent technologies -A mini-review. Front Digit Health. 2022;4:1082884.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Telemedicine and cancer care in India: Promises, opportunities and caveats. Future Sci OA. 2023;8:FSO821.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Role of integrative medicine in the continuum of care of breast cancer patients in the Indian context. Cancer Causes Control. 2021;32:429-40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cost-effectiveness of lifestyle-related interventions for the primary prevention of breast cancer: A rapid review. Front Med (Lausanne). 2020;6:325.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Reasons for non-uptake and subsequent participation in the NHS bowel cancer screening programme: A qualitative study. Br J Cancer. 2014;110:1705-11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Patient use of the internet for health information. Aust Fam Physician. 2014;43:875-7.
- [PubMed] [Google Scholar]
- Evaluation of ‘India against Cancer’ Web Portal Using Environmental Scan and Google Analytics. Int J Sci Res Netw Secur Commun.. 2020;8:14-20.
- [Google Scholar]
- Use of the internet as a health information resource among French young adults: Results from a nationally representative survey. J Med Internet Res. 2014;16:e128.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- e-RUPI Contactless Digital Payment Platform
- Why Breast Cancer Awareness Needs Better Marketing In India. Available from: https://www.forbesindia.com/article/weschool/why-breast-cancer-awareness-needs- better-marketing-in-india/63939/1. [Last accessed 2024 Apr 12].
- Cancer statistics, 2020: Report from national cancer registry programme, india. JCO Glob Oncol. 2020;6:1063-75.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Niramai – A novel breast cancer screening solution. Available from: https://www.niramai.com/. [Last accessed 2024 Apr 12].
- OncoStem Diagnostics Pvt. Ltd. Available from: https://www.oncostem.com. [Last accessed 2024 Apr 12].
- UE LifeSciences. UE LifeSciences. Available from: https://www.uelifesciences.com/. [Last accessed 2024 Apr 12].
- Drug Target Review. Mitra Biotech. Available from: https://www.drugtargetreview.com/company_profile/31020/mitra-biotech/. [Last accessed 2024 Apr 12].
- The Global Breast Cancer Initiative. Available from: https://www.who.int/initiatives/global-breast-cancer-initiative. [Last accessed 2024 Apr 12].
- Estrogen, progesterone and HER2 receptor expression in breast tumors of patients, and their usage of HER2-targeted therapy, in a tertiary care centre in India. Indian J Cancer. 2011;48:391-6.
- [CrossRef] [PubMed] [Google Scholar]
- Access to HER2-targeted therapy at a tertiary care center in India: An evolution. Indian J Cancer. 2022;59:375-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cost effectiveness of Trastuzumab for management of breast cancer in India. JCO Glob Oncol. 2020;6:205-16.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) Ann Oncol. 2015;26:1547-73.
- [CrossRef] [PubMed] [Google Scholar]
- ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol. 2017;28:2340-66.
- [CrossRef] [PubMed] [Google Scholar]
- Profile of pathogenic mutations and evaluation of germline genetic testing criteria in consecutive breast cancer patients treated at a north indian tertiary care center. ann Surg Oncol. 2022;29:1423-32.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Genetic counseling, testing, and management of HBOC in India: An expert consensus document from Indian society of medical and pediatric oncology. JCO Glob Oncol. 2020;6:991-1008.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Statista. India: estimated demand and supply for medical oncologists 2030. Available from: https://www.statista.com/statistics/1376383/india-estimated-demand-and-supply-for-medical-oncologists/. [Last accessed 2024 Mar 24].
- Steps to improve cancer treatment facilities especially radiotherapy infrastructure in India. J Cancer Res Ther. 2022;18:1-4.
- [CrossRef] [PubMed] [Google Scholar]
- Rays of Hope Anchor Centres | IAEA. https://www.iaea.org/services/rays-of-hope/anchor-centres. [Last accessed 2024 Nov 05].





