Translate this page into:
Study of CD4 counts recovery in TB-HIV coinfected patients versus TB uninfected HIV positive patients after (six months) treatment
*Corresponding author: Dr. Shalini Malhotra, Dr Ram Manohar Lohia Hospital, New Delhi, India. drshalinimalhotra@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Kumari R, Roy P, Malhotra S, Kamble U, Kaur N. Study of CD4 counts recovery in TB-HIV coinfected patients versus TB uninfected HIV positive patients after (six months) treatment. Ann Natl Acad Med Sci (India). doi: 10.25259/ANAMS_83_2024
Abstract
Objectives
There is limited research on the recovery of cluster of differentiation 4 (CD4) counts in patients with both human immunodeficiency virus (HIV) and tuberculosis (TB) infections compared to HIV-positive patients without TB following six months of treatment with anti-tuberculosis therapy (ATT) and antiretroviral therapy (ART). This study aims to assess absolute CD4 counts and the percentage of recovery in TB-HIV co-infected patients prior to starting antituberculosis therapy (ATT)+ART, as well as in HIV-positive patients without TB before starting ART. The study will also compare these measurements between the two patient groups after treatment initiation.
Material and Methods
This prospective observational study involved blood sample collection from 50 HIV-positive patients and 50 HIV-positive patients co-infected with tuberculosis before and after the commencement of treatment. CD4 count was performed by flow cytometry. Data was recorded in a Microsoft Excel sheet. Analysis was performed using SPSS version 21.0. Data are presented as numbers and percentages for categorical variables and as mean ± SD and median for continuous variables. Kolmogorov-Smirnov test was used to check normality of the data; if normality was not maintained for any data, non-parametric tests were carried out. Comparisons between groups were performed using unpaired t-tests or Mann-Whitney tests, whereas paired t-tests or Wilcoxon tests were used for comparisons within group comparisons. Qualitative variables were compared using Chi-square tests or Fisher’s exact tests.
Results
The CD4 counts were significantly lower for patients who had both HIV and TB than those with HIV only (p<0.001). The mean rise in CD4 levels at six months of therapy was higher in patients with TB-HIV co-infection when compared to patients without TB at six months (p<0.001). Among the participants, 43 HIV-positive patients and 47 TB-HIV co-infected patients demonstrated CD4 recovery. Out of 50 HIV-TB coinfected patients, 2 patients died and 48 survived, whereas all the 50 patients who had HIV alone survived.
Conclusion
The findings indicate a more significant improvement in CD4 counts among patients with TB-HIV co-infection receiving ATT+ART compared to HIV-only patients on ART. Early detection of tuberculosis in HIV-positive individuals can enhance patient prognosis and reduce mortality rates.
Keywords
Antiretroviral therapy
Antitubercular therapy
CD4 count
HIV
Tuberculosis
INTRODUCTION
Immune deficiency occurs in individuals infected with the human immunodeficiency virus (HIV) as a result of the depletion of cluster of differentiation 4 (CD4) + T lymphocytes.1 Studies indicate that lower CD4 cell counts increase the risk of developing opportunistic infections, especially among HIV positive individuals.1 Out of these, tuberculosis (TB) appears to be the most widespread; it also greatly adds to the burden of morbidity and mortality among HIV positive populations.1
As per the 2020 Global TB Report, the risk of active TB in HIV infected individuals is 18 (15–21) times greater than in HIV-negative individuals, making TB a prevalent cause of mortality in this group.2 CD4 counts give information on antiretroviral therapy (ART).1 Further, CD4 counts are used to guide prophylaxis measures for opportunistic infections and these tests are done on HIV infected individuals every 6 months.1
There is lack of data studying the improvement in CD4 counts in HIV-TB co-infected patients in comparison to TB uninfected HIV positive patients after initiation (6 months) of antituberculosis therapy (ATT) + ART. Hence, this study was carried out to estimate absolute CD4 counts and percentage recovery in TB-HIV coinfected patients versus TB uninfected HIV positive patients before initiation of ATT + ART/ART as well as to compare the CD4 counts and percentage recovery in TB-HIV coinfected patients versus TB uninfected HIV positive patients after (six months) initiation of ATT + ART/ART.
MATERIAL AND METHODS
This prospective observational study was carried out in the Department of Microbiology and the ART Centre at a 1532-bed tertiary care hospital in New Delhi from November 1, 2017, to March 31, 2019. Blood samples were collected at the Microbiology Department Integrated Counseling and Testing Centre (ICTC) from ART/ATT naïve (not on ART and ATT) HIV-positive patients who were coinfected with TB and TB uninfected HIV positive patients attending the hospital. We calculated a sample size of 50 in each group with a power of 90%, given the existing literature based on the study by Wanchu et al.3 (2010). Fifty HIV-positive patients and 50 HIV-positive patients coinfected with TB were taken as study groups. Drug compliance was ensured with regular follow-up of the included patients whenever they came to ART center and also telephonically. First-line drug regimen was started, that is, tenofovir, lamivudine, efavirenz in all the included patients; none of the patients developed any side effects with this regimen. ART was started after two weeks of ATT.
Inclusion criteria
-
1.
Newly detected TB-HIV coinfected adult (age 18–60 years) patients of either gender who are not previously treated and recently diagnosed with this coinfection.
-
2.
Newly detected HIV-positive adult (age 18–60 years) patients of either gender who have not been treated yet.
Exclusion criteria
-
1.
Patients who present with coinfection other than TB, such as fungal infection, Pneumocystis jiroveci pneumonia, Hepatitis, Syphilis, and more. Patients with any other comorbidities were excluded to rule out any confounding factors.
Venous blood, 3–3.5 mL in volume, was collected aseptically in BD EDTA (Becton Dickinson ethylenediaminetetraacetic acid) vial for CD4 counts. First sample was collected before starting the treatment (ATT + ART/only ART). The second sample was collected six months after initiation of treatment (ATT + ART/only ART). CD4 count was performed by flow cytometry using FACSCalibur (manufacturer: Becton-Dickinson) as per the manufacturer’s instructions. A minimum of 100 µL of whole blood was used to determine the percentage and absolute count of helper T lymphocytes in whole blood. The software identifies the lymphocyte population of interest and calculates absolute CD4 count and CD4 percentage automatically.
Test procedure
BD Tritest CD3/CD4/CD45 reagent, 20 µl in volume, was added to a BD Trucount tube. Following this, 50 µl of well-mixed anticoagulated whole blood was introduced in the tube. After this, the tube was capped and gently vortexed to ensure proper mixing. It was followed by incubation in the dark for 15 minutes. Following this incubation, 450 µl of 1X BD FACS lysing solution was added, and the mixture was vortexed once more before undergoing another 15-minute incubation. Once again, the mixture was vortexed and then loaded into racks for analysis using the BD FACSCalibur.
CD4 counts, expressed as cells/microliter, were recorded for all participants, including those with HIV alone and those coinfected with HIV and TB.
Statistical analysis
Data was analyzed using SPSS version 21.0. Categorical variables were presented as numbers and percentages (%). Continuous variables were presented as mean ± SD and median. The Kolmogorov-Smirnov test was employed to check the normality of the data; failing its parameters prompted nonparametric tests.
The following statistical tests were applied:
-
1.
Quantitative variables between the two groups were compared using the unpaired t-test. Mann-Whitney test was used for data which were not normally distributed. The paired t-test or Wilcoxon test was used for comparisons within groups over time.
-
2.
Qualitative variables were analyzed by the Chi-squared test or Fisher’s exact test, as suitable.
RESULTS
During the study period, from the total 100 patients included in the study, 2 patients died who were HIV positive and coinfected with TB, and 98 patients survived. Out of the 100 study patients, 50 had HIV-TB coinfection while 50 were suffering from HIV alone. The two patients who died had a very low CD4 count (10 cell/µL and 15 cell/µL, respectively) and severe weight loss. One died of cardiac arrest, while the other had respiratory failure.
Symptomatic distribution
It was observed that most of the patients in the study presented with symptoms of fever (48%), cough (13%), and diarrhea (11%). Other symptoms that were less commonly seen included weight loss (8%), body rash (5%), abdominal pain (4%), fatigue (4%), weakness (3%), body ache (2%), and dyspnea and loss of appetite (1%) [Figure 1].
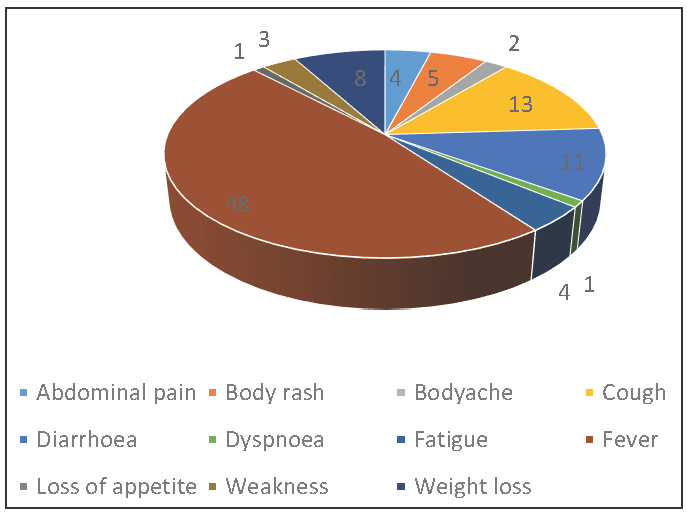
- Symptomatic distribution of patients in the study group.
Demographic Profile
Demographic profile of all the patients in the study group showed that the mean age of patients was 31.98 years, with a minimum age of 19 years and a maximum age of 57 years. The mean age of HIV alone and HIV + TB patients was 29.6 years and 34.36 years, respectively (p = 0.001 by T-test).
Mean weight of the patients was found to be 51.99 kg, with 30 kg minimum and 75 kg maximum. The standard deviation of the age of the patients was 7.172 and for weight of the patients was 10.903. The mean weight of HIV alone and HIV + TB patients was 55.68 kg and 48.3 kg, respectively (p = 0.001 by T-test).
Pattern of CD4 counts
Out of the total patients studied, the CD4 counts ranged from 10 to 900 cell/µL, with a mean of 233.73 cell/µL before starting the treatment. After six months of treatment, the mean of CD4 counts were 400.17 cell/µL, with minimum CD4 counts at 98 cell/µL and maximum CD4 count at 1029 cell/µL. The standard deviation of patients before starting the treatment was 186.56, and after six months of treatment was 192.20.
Gender wise distribution of patients according to their infection
The gender distribution of the 100 patients was examined, revealing that 70 were male and 30 female. Among the male patients, 33 were diagnosed with HIV alone while 37 were coinfected with HIV and TB. For the female patients, 17 had only HIV and 13 were coinfected with both HIV and TB. The chi-squared test yielded a p-value of 0.383, indicating no statistically significant difference between the two genders.
Mortality pattern of patients according to their infection
When we observed the mortality pattern amongst the study population, we found that out of the 50 HIV-TB coinfected patient, 2 patients died and 48 survived, whereas all the 50 patients who had HIV alone survived.
Symptomatic distribution of patients according to their infection
Considering the symptomatic distribution of patients with HIV/HIV-TB coinfection, we found that there was a significant difference in the symptom of cough between these two groups of patients (p = 0.0001). Out of 50 patients coinfected with TB, 26% patients presented with the symptom of cough, while none of the patients with HIV alone had a cough.
Out of the 50 HIV alone positive patients, 48% patients presented with the symptom of fever, and similar percentage was seen in the TB coinfected patients. Diarrhea was also an important symptom, which was found in 8% of HIV positive patients and in 14% of patients who were coinfected with TB [Table 1].
| Persistent symptoms | Group | Total | P-value (Fisher’s exact test) | |
|---|---|---|---|---|
| HIV alone | HIV + TB | |||
| Abdominal pain | 1 | 3 | 4 | 0.62 |
| 2.0% | 6.0% | 4.0% | ||
| Body rash | 5 | 0 | 5 | 0.056 |
| 10.0% | .0% | 5.0% | ||
| Body ache | 2 | 0 | 2 | 0.494 |
| 4.0% | .0% | 2.0% | ||
| Diarrhea | 4 | 7 | 11 | 0.525 |
| 8.0% | 14.0% | 11.0% | ||
| Fatigue | 4 | 0 | 4 | 0.118 |
| 8.0% | .0% | 4.0% | ||
| Fever | 24 | 24 | 48 | 1.000 |
| 48.0% | 48.0% | 48.0% | ||
| Weakness | 3 | 0 | 3 | 0.242 |
| 6.0% | .0% | 3.0% | ||
| Weight loss | 1 | 7 | 8 | 0.0590 |
| 2.0% | 14.0% | 3.0% | ||
| Cough | 0 | 13 | 13 | 0.0001 |
| 0% | 26.0% | 13.0% | ||
| Dyspnea | 0 | 1 | 1 | 1.000 |
| 0% | 2.0% | 1.0% | ||
| Loss of appetite | 0 | 1 | 1 | 1.000 |
| 0% | 2.0% | 1.0% | ||
| Total | 50 | 50 | 100 | |
| 100.0% | 100.0% | 100.0% | ||
HIV: Human immunodeficiency virus, TB: Tuberculosis
Type of tuberculosis
Out of total 50 TB infected patients, 35 patients had pulmonary TB and 15 patients had extrapulmonary TB. Out of the 15 extrapulmonary TB cases, five patients had lymph node TB and the remaining ten patients had abdominal TB.
Mortality pattern of patients in HIV-TB coinfected group
When we analyzed the mortality pattern of TB coinfected patients, we found that out of 35 patients who had pulmonary TB coinfection, two died and none died from the extrapulmonary TB coinfection.
The fisher’s exact test for these was p = 1.00, so no significant difference was observed in the mortality pattern.
Pattern of CD4 counts in patients
The CD4 counts were compared before initiation of treatment between HIV/HIV-TB coinfected patient using T test method. HIV-TB coinfected patients significantly (p < 0.001) had lower CD4 count in comparison to HIV alone positive patients, whereas when we compared the mean of CD4 counts after six months of treatment between HIV/HIV-TB coinfected patients, no significant difference was obtained (p = 0.486) [Table 2].
| Variables | Group | N | Mean | Standard deviation | T value | Mann-Whitney U |
|---|---|---|---|---|---|---|
| CD4 before ART | HIV ALONE | 50 | 330.42 | 207.559 |
6.041 P < 0.001 |
-5.195 P < 0.001 |
| HIV + TB | 50 | 137.04 | 90.319 | |||
| CD4 after six months of ART | HIV ALONE | 50 | 422.54 | 226.237 |
1.178 P = 0.242 |
-0.696 P = 0.486 |
| HIV + TB | 48 | 376.88 | 147.644 |
CD4: Cluster of differentiation 4, ART: Antiretroviral therapy, HIV: Human immunodeficiency virus, TB: Tuberculosis
Comparison of changes in CD4 count
However, when we compared the mean of changes in the CD4 count before and after six months of treatment, we found a significant difference between HIV alone and TB-HIV coinfected patients. The changes in the CD4 counts were significantly (p < 0.001) higher in HIV-TB coinfected patients. Mean of CD4 count change in HIV/TB coinfected patient was 234.64, whereas it was 92.12 in HIV positive patients [Table 3].
| Variables | Group | N | Mean | Standard deviation | T value | Mann-Whitney U |
|---|---|---|---|---|---|---|
| CD4 change | HIV ALONE | 50 | 92.1200 | 122.73692 |
-5.544 P < 0.001 |
-4.964 P < 0.001 |
| HIV + TB | 48 | 234.6458 | 131.72384 |
CD4: Cluster of differentiation 4, ART: Antiretroviral therapy, ATT: Antituberculosis therapy, HIV: Human immunodeficiency virus, TB: Tuberculosis
Recovery patterns in terms of CD4 count
Out of 50 HIV positive patients, 43 patients recovered, that is, they showed improvement in their CD4 counts, whereas among 50 HIV-TB coinfected patients, 47 recovered. Seven HIV alone and three TB-HIV coinfected patients showed decline in their CD4 counts despite ART/ATT + ART. The chi-squared test gives a p-value of 0.182, which is not a significant difference between HIV alone and TB-HIV coinfected patients’ recovery.
DISCUSSION
HIV-TB coinfection poses a significant health challenge, particularly in developing countries like India. Treating TB in conjunction with HIV can enhance the immune function of coinfected patients.4 In this research, we compared the changes in CD4 count after six months of ATT + ART/ART in TB-HIV coinfected patients and TB uninfected HIV positive patients, respectively.
Considering the total study group of 100 patients, the male to the female ratio was found to be 2.33:1. In HIV alone positive patients, 66% were males and 34% females, while in TB-HIV coinfected patients, 74% were males and 26% females (p-value of 0.383 by chi-squared test). In this study, we found male preponderance in both HIV alone and TB-HIV coinfected patients. Similar results were obtained in another study conducted in Northeast India where the preponderance of males were found.5 In concordance with this study, the Northeast India study also showed an insignificant p-value 0.16 for the difference in male to female ratio. Furthermore, a 2011 European study highlighted that TB-HIV coinfection was more prevalent in males, while a Brazilian study indicated a significant difference favoring males (p < 0.001).6,7
The increased prevalence of TB-HIV coinfection in males may be linked to their higher likelihood of migrating for work, which results in greater exposure to Mycobacterium tuberculosis. Additionally, it’s important to highlight that the reactivation of TB is particularly frequent among individuals with HIV/AIDS.8
The mean age of TB-HIV coinfected patients was 34.36 years compared to 29.60 years in HIV patients. This age difference was statistically significant by T-test (p = 0.001). Similar findings have been reported in studies from Northeast India and Karnataka, indicating that TB-HIV coinfection tends to occur more frequently in older age groups.5,9 A Nigerian study from 2014 found that TB-HIV coinfection was 2.7 times more prevalent in patients over 40 years old than in those under 25.10 Moreover, research from South Sudan noted a significant majority of HIV/TB coinfected patients in the 25–34 age bracket, a group that often bears familial responsibilities, thus increasing their likelihood of interacting with infected individuals.8
Mean weight of the patients who had HIV alone and who had TB-HIV coinfection was 55.68 years and 48.30 years, respectively. The difference was statistically significant with p = 0.001. Screening symptoms for TB, mainly in HIV-positive patients are fever, night sweat, cough, and weight loss. Weight loss is a major screening symptom of TB.1 A study from Brazil showed a higher frequency of weight loss, that is, >10 kg in TB-HIV coinfected patients.11
Out of the 100 study patients, two patients died and the rest survived during the study period. The two patients who died had TB-HIV coinfection (pulmonary TB). However, the results were not significant (p = 0.49). Our findings align with a study from Manipur that reported a predominance of pulmonary TB over extrapulmonary forms in HIV-positive patients, a trend also observed in African research.12,13
In concordance with this study, several studies showed similar pattern of mortality, that is, high mortality in TB-HIV coinfected patients.14–19 Both conditions that is, TB and HIV, accelerate the progression of each other and affect the cell-mediated immune system. In individuals with compromised immune systems, such as those living with HIV, the typical progression of TB is altered.20 Latency period between infection and the onset of disease shortens, and those with HIV may develop active TB within just weeks to months as opposed to the usual timeline of years or even decades.20
Analysis of the CD4 counts shows that the mean before starting ART in HIV alone patients was 330.42 and in TB-HIV coinfected patients, the mean before starting ATT + ART was 137.04. The findings were in correlation with the Centers for Disease Control and Prevention (CDC) classification of opportunistic infections in HIV in relation to CD4 counts, as per which TB occurs when CD4 counts are below 200 cells/µL.1,21 The difference by both T-test and Mann-Whitney test between the mean of CD4 counts of HIV alone and TB-HIV coinfected patients was statistically significant (p = 0.001). A Nigerian study also found that TB as an opportunistic infection occurs when CD4 counts fall below this threshold.21 Another study from Ethiopia in 2020 reported a high incidence of TB in HIV-positive patients, especially in patients with CD4+ T cell count < 200 cells/µL.22
The mean of CD4 counts after six months of ART in HIV alone patients was 422.54 cells/µL, while in TB-HIV coinfected patients, the mean was 376.88 cells/µL after six months of ATT + ART. But the difference was statistically insignificant (p-value of 0.242 and 0.486 by T-test and Mann-Whitney test, respectively). This may be due to the greater increase in CD4 counts after six months of ATT + ART in patients who were coinfected with TB in comparison to TB-uninfected HIV patients who were on ART alone.
The mean of changes in CD4 counts in HIV alone patients was 92.12 and the mean of changes in CD4 counts in TB-HIV coinfected patients was 234.64; so, there was less improvement in CD4 counts in HIV alone patients who were on ART in comparison to the TB-HIV coinfected patients who were on ATT + ART. When we compared the mean of changes in CD4 counts, we found that it was statistically significant with p-value < 0.001 by both T test and Mann-Whitney test. Further increase in CD4 count in patients with TB coinfection following treatment hints that the immune suppression observed at the onset of TB may result from both mycobacterial growth and the interaction between TB and HIV besides the effect of HIV alone. In correlation with this study, one more study conducted in 2010 in India also reported that there is a greater improvement in CD4 counts in HIV patients who are coinfected with TB and receive ATT + ART in comparison to HIV alone positive patients who receive only ART.3 Reduction in CD4 lymphocytes is known to occur in HIV negative TB patients and become normalized following ATT.23–27 So, ATT also leads to improvement in CD4 counts in TB-HIV coinfected patients. In a study involving 85 patients with TB who were not infected with HIV, 37 exhibited low CD4 cell counts, while 48 had normal CD4 levels.28
Research on patients with dual infections who were not receiving ART has reported varying outcomes regarding changes in CD4 counts after undergoing ATT.28–31 In concordance to this study, a South African study showed the improvement in CD4 counts in TB-HIV coinfected patients following ATT, but the change in CD4 counts was not statistically significant.29 A study conducted in Kampala, Uganda, involving HIV-positive patients with pleural TB, indicated an improvement in CD4 counts after ATT, although the change was not statistically significant.30 The observed increase in CD4 counts following both ATT and ART suggests that immune reconstitution may occur after ART, especially in patients with initially low CD4 levels.3
Considering the recovery of patients out of the 50 HIV alone patients included in this study, 43 recovered, that is, they showed improvement in their CD4 counts after six months of ART, and seven did not recover. These seven patients showed a decline in their CD4 counts after six months of ART. Out of the 50 TB-HIV coinfected patients, 47 recovered, that is, they showed improvement in CD4 counts after six months of ATT + ART. So, 86% patients who had HIV alone and 94% patients who had TB-HIV coinfection showed improvement in their CD4 counts, but the difference was not statistically significant (p = 0.182 by chi-squared test). Those patients who did not show improvement in their CD4 counts were further tested for viral load. The viral load was found to be high in these patients and the patients were then put on second-line treatment. So, the reason for the treatment failure in these patients may be drug resistance.
The small sample size of our study is the major limitation of the study. Other limitations are that HIV viral load was not done for all the patients to rule out confounding factors and patients with other comorbidities were not included. Future research should consider a larger prospective study with distinct groups for TB only, HIV only, and TB-HIV coinfection to validate these findings and explore CD4 count improvements further. Hence, more studies are needed to see the pattern of CD4 counts improvement in TB-HIV coinfected patients in comparison to HIV alone patients.
CONCLUSION
This study indicates that TB-HIV coinfected patients treated with the combined ATT and ART experience showed greater improvements in CD4 counts compared to HIV-only patients receiving ART. We infer that in HIV/TB coinfected individuals, TB additionally contributes to a reduction in CD4 counts. So, the early diagnosis and treatment of TB in HIV-positive patients could enhance patient prognosis and reduce mortality rates.
Authors’ contributions
RK, PR, SM, UK, NK: Contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work, drafting the work or revising it critically for important intellectual content. Final approval of the version to be published. Agreement to be accountable for all aspects of the work to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical approval
The research/study approved by the Institutional Review Board at Atal Bihari Vajpayee Institute of Medical Sciences (ABVIMS) and Dr. Ram Manohar Lohia Hospital, Delhi, number F. No. TP(MD/MS)(31/2017)IEC/PGIMER/RMLH 1743/17, dated 30th October 2017.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Human immunodeficiency virus disease: AIDS and other related disorders. In: Loscalzo J, Kasper DL, Longo DL, Fauci AS, Hauser SL, Jameson JL, eds. Harrison’s principles of internal medicine (21 edition). New York: McGraw Hill; 2022. p. :1527-96.
- [Google Scholar]
- Tuberculosis and HIV. World Health Organization. 2020. https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/treatment/tuberculosis-hiv [Last accessed 2024 Nov 25].
- CD4 cell count recovery in HIV/TB co-infected patients versus TB uninfected HIV patients. Indian J Pathol Microbiol. 2010;53:745-9.
- [CrossRef] [PubMed] [Google Scholar]
- Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;43:42-6.
- [CrossRef] [PubMed] [Google Scholar]
- Study on HIV-TB coinfection among patients attending a tertiary care centre in North East India. Int J Health Sci Res. 2017;7:94-101. https://www.ijhsr.org/IJHSR_Vol.7_Issue.6_June2017/15.pdf
- [Google Scholar]
- Tuberculosis and HIV co-infection in European Union and European economic area countries. Eur Respir J. 2011;38:1382-92.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics of pulmonary tuberculosis in HIV seropositive and seronegative patients in a Northeastern region of Brazil. Rev Soc Bras Med Trop. 2004;37:46-50.
- [CrossRef] [PubMed] [Google Scholar]
- HIV and TB co-infection in South Sudan: a three year retrospective study. South Sudan Med J. 2014;7(4):86-90.
- [Google Scholar]
- CD4 count evaluation in HIV-TB co infection before and after anti-tubercular treatment. Int J Res Med Sci. 2014;2:1031-4.
- [CrossRef] [Google Scholar]
- Factors associated with TB/HIV co-infection among drug sensitive tuberculosis patients managed in a secondary health facility in Lagos, Nigeria. Afr J Infect Dis. 2017;11:75-82.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Characteristics of pulmonary tuberculosis in HIV seropositive and seronegative patients in a Northeastern region of Brazil. Rev Soc Bras Med Trop. 2004;37:46-50.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of extrapulmonary tuberculosis among people living with HIV/AIDS in sub-Saharan Africa: A systemic review and meta-analysis. HIV AIDS (Auckl). 2018;10:225-37.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181:80-6.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of death among TB/HIV co-infected patients on tuberculosis treatment in Sichuan, China: A retrospective cohort study. Medicine (Baltimore). 2023;102:e32811.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- [Effect of antiretroviral therapy in reducing deaths among patients co-infected with Mycobacterium tuberculosis and human immunodeficiency virus in Guangxi] Zhonghua Liu Xing Bing Xue Za Zhi. 2015;36:124-7.
- [PubMed] [Google Scholar]
- The role of social determinants on tuberculosis/HIV co-infection mortality in southwest Ethiopia: A retrospective cohort study. BMC Res Notes. 2016;9:89.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Beneficial effect of isoniazid preventive therapy and antiretroviral therapy on the incidence of tuberculosis in people living with HIV in Ethiopia. PLoS One. 2014;9:e104557.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antiretroviral therapy at a district hospital in Ethiopia prevents death and tuberculosis in a cohort of HIV patients. AIDS Res Ther. 2006;3:10.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Information about tuberculosis. GHE 2022. www.tbfacts.org.
- Assessment of total serum immunoglobulin Levels and CD4 T-Lymphocyte counts in pulmonary tuberculosis patients co-infected with HIV in Uyo, Nigeria. J Adv Med Res. 2017;22:1-9.
- [Google Scholar]
- Tuberculosis and its association with CD4+ T cell count among adult HIV positive patients in Ethiopian settings: A systematic review and meta-analysis. BMC Infect Dis. 2020;20:325.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- T4 lymphopenia in patients with active pulmonary tuberculosis. Clin Exp Immunol. 1985;60:49-54.
- [PubMed] [PubMed Central] [Google Scholar]
- T4 lymphopenia in human tuberculosis. Tubercle. 1987;68:195-200.
- [CrossRef] [PubMed] [Google Scholar]
- Normalization of CD4+ T-lymphocyte depletion in patients without HIV infection treated for tuberculosis. Chest. 1994;105:1335-7.
- [CrossRef] [PubMed] [Google Scholar]
- CD4 cell counts in human immunodeficiency virus-negative patients with tuberculosis. Clin Infect Dis. 1997;24:988-91.
- [CrossRef] [PubMed] [Google Scholar]
- CD4+ T-lymphopenia in HIV negative tuberculous patients at King Khalid University Hospital in Riyadh, Saudi Arabia. Eur J Med Res. 2011;16:285-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Long term follow up of HIV-infected patients with tuberculosis treated with 6-month intermittent short course chemotherapy. Natl Med J India. 2008;21:3-8.
- [PubMed] [Google Scholar]
- Human immunodeficiency virus-1 RNA levels and CD4 lymphocyte counts, during treatment for active tuberculosis, in South African patients. J Infect Dis. 2003;187:1967-71.
- [CrossRef] [PubMed] [Google Scholar]
- A Randomized, double-blind, placebo-controlled trial of the use of prednisolone as an adjunct to treatment in HIV-1—associated pleural tuberculosis. J Infect Dis. 2004;190:869-78.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in HIV RNA viral load, CD4+ T-cell counts, and levels of immune activation markers associated with anti-tuberculosis therapy and cotrimoxazole prophylaxis among HIV-infected tuberculosis patients in Abidjan, Cote d’Ivoire. J Med Virol. 2005;75:202-8.
- [CrossRef] [PubMed] [Google Scholar]





