Translate this page into:
Small steps, big impact: Effect of mandatory sputum quality assessment prior to sputum culture processing on quality of samples received
* Corresponding author: Dr. Nisha Goyal, MBBS, MPH, MD, Department of Microbiology, University College of Medical Sciences & Guru Teg Bahadur Hospital, Delhi, India. drnishagoyalucms@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Goyal N, Jahan R, Varshney A, Banik D, Gupta A, Kaushal A, et al. Small steps, big impact: Effect of mandatory sputum quality assessment prior to sputum culture processing on quality of samples received. Ann Natl Acad Med Sci (India). doi: 10.25259/ANAMS_217_2024
Abstract
Objectives
Unnecessary culturing of poor-quality sputum samples may lead to wastage of precious resources. This study highlights changes in the overall quality of sputum samples received in the lab after the implementation of mandatory sputum sample quality assessment by direct microscopy and the subsequent culture processing of only good-quality sputum samples.
Material and Methods
Quality of sputum was assessed according to widely used standard criteria and only sputum samples with Bartlett’s score of +1 or more were cultured. Assessment of sputum sample quality improvement was done over a six-month study period.
Results
A total of 1237 sputum samples were collected over six months. In the first month, out of the 163 sputum samples processed, only 41 (24%) were valid by the standard criteria. In the second month, 55% were valid, and in the third month, this percentage further increased to 67%. This percentage further increased in the following months, with over 70% of samples found to be valid in the last two consecutive months. The p-value was found to be significant (p<0.05).
Conclusion
Rampant antibiotic prescriptions for bacterial isolates from these poor-quality samples further contribute to the emergence of antimicrobial resistance (AMR) besides the wastage of resources. This study emphasizes the urgent need for stricter execution of the rejection policy of poor-quality sputum samples.
Keywords
Bartlett scoring
LRTI
sputum culture
sputum quality
sputum quality assessment
INTRODUCTION
Lower respiratory tract infections (LRTIs) are the most prevalent medical conditions that necessitate frequent hospitalization and consultation. According to Carroll (2002), LRTIs are among the most prevalent infectious disorders affecting people globally.1 The microscopic examination of sputum and its culture are often utilized laboratory techniques for LRTI diagnosis. Commonly, the deeply coughed early morning sputum samples are collected to avoid bias in the interpretation of the results. The quality of the sputum sample received in the laboratory is crucial in finding the causative bacteria and further helping guide definitive antibiotic therapy.
Despite being a valuable sample for the diagnosis of LRTIs, the scarcity of a standard protocol often leads to erroneous results in diagnosis and causes delays in initiating empirical antimicrobial therapy. People of extremes of age (elderly and pediatric population), often fail to produce deep cough. In the absence of technical expertise, induced expectorations are not often collected in several clinical settings, even when necessary. Sputum samples received in microbiological laboratories are often watery in nature which raises the suspicion of contamination with saliva. The presence of saliva increases the chance of contamination with normal flora of the oropharynx (upper respiratory tract secretions).2 Sputum quality assessment is a useful tool for distinguishing the true respiratory pathogens from possibly colonizing flora. Most of the low-quality sputum samples result in a negative culture report. It should be reported with the remark that ‘the specimens were of low quality,’ so the possibility of false negative results is not excluded. However, the biggest concern in these cases is the culture-positive specimens (among the non-acceptable category) where the majority of isolated bacteria are usually found to be multidrug-resistant (MDR) bacteria, such as extended spectrum beta-lactamase-producing Escherichia coli and Methicillin-Resistant Staphylococcus aureus.3 Evaluation of sputum quality before further processing has the advantage of aiding in the detailed interpretation of the sputum culture and sensitivity report. Moreover, this helps the clinicians to choose the right antibiotics, which can further prevent and reduce the occurrence of antimicrobial resistance.
Assessment of the sputum quality allows us to estimate the amount of oropharyngeal contamination. This method is performed by microscopic examination of the cellular components in a stained smear of the sputum specimen that is seen under low power field (LPF) magnification. The presence of two cell types: (i) squamous epithelial cells (SECs) and inflammatory cells, (ii) primarily polymorphonuclear leukocytes, is taken into consideration. SECs are found only in the upper respiratory tract, suggesting oropharyngeal contamination, whereas the presence of polymorphonuclear leukocytes suggests material derived from the site of active infection.4
There are several published criteria for assessing the quality of sputum. According to Murray and Washington (1975) sputum quality assessment should be dependent only on the presence of the SECs that are seen microscopically at LPF magnification.5 Alternatively, Van Scoy states that sputum specimens with more than 25 leukocytes per LPF should be processed further.6 When only one type of cell is taken into consideration, the variations in the thickness of the material in different areas of the same slide can cause inconsistencies. This difficulty can be avoided by assessing sputum quality according to the white blood cells-SEC ratio, as Bartlett’s recommendation.7 In this study, we aim to highlight the importance of accepting or rejecting a sputum sample based on quality assessment by direct microscopy according to the criteria given by Bartlett et al.7 (1998)
MATERIAL AND METHODS
A prospective analytical study was conducted in the bacteriology laboratory, Department of Microbiology from a healthcare institute in Delhi over six months. The study included all the sputum specimens from suspected LRTI cases received in the Microbiology lab for routine bacteriological processing. The standard laboratory protocols were followed for the processing of these samples. A purulent portion of samples was used for making smears for Gram staining and inoculating culture media. Results of Gram-stained smears were interpreted based on the presence of microorganisms, pus cells, and epithelial cells, as seen under a microscope.
The quality of sputum was assessed according to the criteria of Bartlett et al.7 (1998), based on the relative number of SEC and inflammatory cells seen microscopically at LPF magnification and scores were assigned [Table 1].
| Type of cells | Number of cells per low power field (scanned at 10x) | Score |
|---|---|---|
| Number of neutrophils | <10 | 0 |
| 10-25 | +1 | |
| >25 | +2 | |
| Number of squamous epithelial cells | <10 | 0 |
| 10-25 | -1 | |
| >25 | -2 |
Total score of +1 or more indicates good sputum quality.
According to these criteria, for every specimen, a Q-score, which was the sum of “+” and “– “assigned values, was calculated. The “+” Q-score indicated material derived from the site of an active infection, and these samples were categorized as acceptable. A “0” or “-” Q-score suggested low sputum quality and excessive oropharyngeal contamination, and these samples were categorized as non-acceptable.7 Only the good-quality sputum samples with a final Q score of >0 were further cultured for bacterial pathogen isolation.
RESULTS
A total of 549 sputum samples were collected over six months (April-September 2023). All samples were assessed for adequacy by Bartlett’s criteria [Table 1] for sputum assessment.
The percentage of good-quality sputum samples steadily increased over the course of the study as written feedback to clinicians regarding the Bartlett score of the given samples continued [Figure 1]. In April, a total of 163 sputum samples were processed, out of which only 41 (24%) were found to be valid. The total number of samples collected in the next month was 201, of which 112 (55%) were found to be valid. This percentage further increased in the following months, with over 70% of samples found valid in the last two consecutive months. The p-value was found to be <0.05 which is significant.
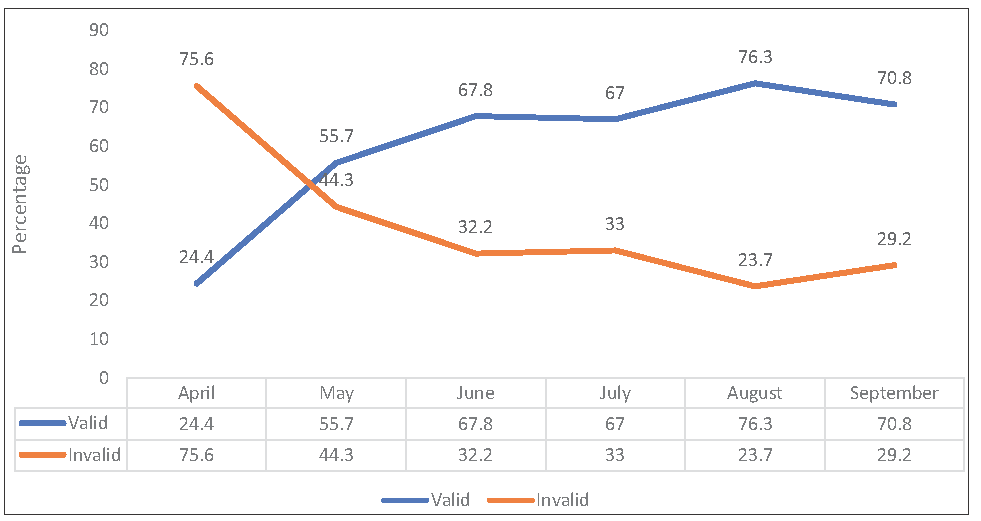
- Distribution of quality of sputum samples received after implementation of mandatory sputum quality assessment prior to culture processing over the study period. Valid denotes Bartlett’s score of +1 or more; invalid denotes Bartlett’s score of ≤0.
The majority of sputum samples were received from the medicine department (92%), followed by the Obstetrics & Gynaecology department. Department-wise distribution of sputum samples received has been shown in Table 2.
| Department | Frequency distribution n (%) |
|---|---|
| Medicine | 1134 (91.68%) |
| Surgery | 20 (1.62%) |
| Obstetrics & gynecology | 53 (4.28%) |
| ENT | 12 (0.97%) |
| Orthopedics | 5 (0.4%) |
| Others | 13 (1.05%) |
| Total | 1237 (100%) |
ENT: Ear, Nose, Throat
Overall distribution of sputum samples from male (618/1237) and female (619/1237) patients was similar. However, the percentage of valid samples received from female patients (50.4%) was marginally better than samples received from male (49.6%) patients. There was no significant difference in the validity of samples with respect to the sex of the patient. The month wise distribution of samples received from each sex has been shown in Figure 2.
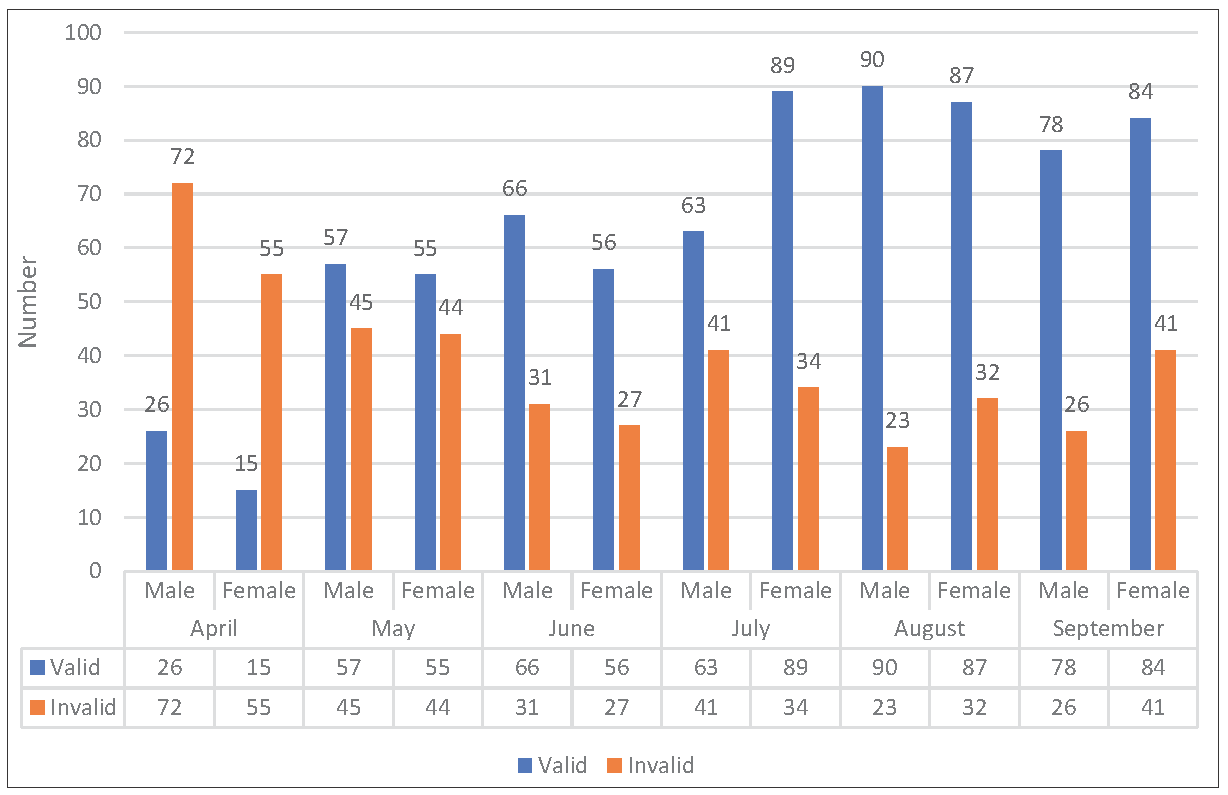
- Gender-wise distribution of validity of sputum samples after implementation of mandatory sputum quality assessment prior to culture processing over the duration of study period.
With respect to age, 62.2% (643/1033) of valid samples were collected from adult patients aged between 19-59 years of age, whereas the validity of samples collected from elderly patients and children below the age of 18 years was 57.2% (107/187) and 94% (16/17), respectively [Table 3].
| Age | Bartlett’s score of ≥1 (Valid) | Bartlett’s score of ≤0 (Invalid) | Total |
|---|---|---|---|
| Elderly >60 years | 107 | 80 | 187 |
| Adults <60 years | 643 | 390 | 1033 |
| Children <12 years | 16 | 1 | 17 |
Tables 4 and 5 show the distribution of the culture-positivity of sputum samples among valid (Bartlett’s score of ≥1) and invalid (Bartlett’s score of ≤0) Barlett scores. Isolation of pathogenic bacteria was higher among the sputum samples with Bartlett’s score of ≥1 in comparison to samples with Bartlett’s score of ≤0.
| Month | Percentage of culture-positive samples (%) | |
|---|---|---|
| Bartlett’s score of ≥1 (Valid) | Bartlett’s score of ≤0 (Invalid) | |
| April | 22 | 3 |
| May | 1.78 | 0 |
| June | 13.9 | 1.7 |
| July | 16.45 | 2.66 |
| August | 6.2 | 0 |
| September | 9.25 | 0 |
| Total | 10.3 | 1.5 |
| Month | Bartlett’s score of ≥1 (Valid) | Bartlett’s score of ≤0 (Invalid) | ||||
|---|---|---|---|---|---|---|
| NPO | Pathogen isolated | Total number of valid samples | NPO | Pathogen isolated | Total number of invalid samples | |
| April | 32 | 9 | 41 | 123 | 4 | 127 |
| May | 110 | 2 | 112 | 89 | 0 | 89 |
| June | 105 | 17 | 122 | 57 | 1 | 58 |
| July | 127 | 25 | 152 | 73 | 2 | 75 |
| August | 166 | 11 | 177 | 55 | 0 | 55 |
| September | 147 | 15 | 162 | 67 | 0 | 67 |
| Total | 687 | 79 | 766 | 464 | 7 | 471 |
NPO: No pathogen isolated.
DISCUSSION
Isolation of the pathogenic organism of a particular infection from the samples obtained by the clinician defines the work of a medical microbiologist. Collection of good quality sputum samples depends on the healthcare workers’ training and their ability to correctly communicate to and instruct the patient as well as the patient’s cooperation throughout the entire collection process.8
During this six-month period of our study, we gave feedback to clinicians with their Sputum culture reports in the form of Bartlett’s score and its validity criteria. This seems to have improved the sputum quality throughout the study, as the percentage of good-quality sputum samples received has significantly increased.
An acceptable monthly rate of poor-quality sputum is 25% of the total sputum specimens.9 If the percentage of poor-quality specimens exceeds the threshold limit for three consecutive months, evaluation and specimen collection re-training must be carried out. Thus, assuring the quality of the sputum sample before processing is relevant.10
At the beginning of our study, 75% of the sputum specimens obtained were of poor quality. Despite the regular feedback from the clinician, 29% samples were still of poor quality in the last month of our study. In the context of a large laboratory, processing hundreds of samples daily, these percentages turn out to be huge in numbers. Therefore, the rejection of such a huge number of specimens tends to be complex. Also, if low-quality specimens are subjected to bacterial identification processes, then laboratory resources, including personnel and reagents are also wasted.
Reporting of poor-quality samples should be modified accordingly, with the added justification that the isolated bacteria are likely a part of the colonizing flora. Additionally, automatic reporting of AST values could be avoided because MDR-organisms isolated from poor-quality sputum result in the overprescription of antibiotics, a problem that should be solved by the collaborative efforts of doctors and microbiologists.11 The importance of microorganisms recovered from respiratory samples must always be evaluated in light of clinical history.12
The results of our study indicate that the collection of poor-quality sputum samples will always remain a common problem faced by microbiologists. Therefore, it is necessary to improve our capability for good sputum sample collection.13 There is an urgent need for stricter execution of operational procedures for standard sputum collection and processing. Receiving a good quality sputum sample is necessary to isolate pathogenic organisms from culture and subsequently diagnose and treat LRTIs correctly.14
In a study done by Deris ZZ et al. in 2008 to determine the usefulness of the application of Bartlett criteria during the processing of sputum samples, they concluded that the introduction of Bartlett’s criteria was able to reduce unnecessary processing of sputum specimens and significantly increase the isolation of respiratory pathogens in clinical specimens.15 During their study over a 2-year period, the percentage of total sputum samples processed, based on Bartlett criteria, decreased in 2 years but the percentage of specimens growing significant pathogens increased. The isolation of normal upper respiratory tract flora also is reduced.
CONCLUSION
In a high-volume center with limited manpower and logistics. It becomes crucial that we utilize the available resources at hand judiciously. An unnecessary culture of poor-quality sputum samples may lead to the wastage of precious human effort and resources. Therefore, it is of paramount importance that the quality of sputum samples should be ascertained before processing them any further.
Author’s contributions
NG, RJ, AV, DB, AG, AK,NPS : Conception and design, acquisition of data, or analysis and interpretation of data; drafting the article; final approval of the version to be published; SD: Acquisition of data, or analysis and interpretation of data; drafting the revised article; final approval of the version to be published.
Ethical approval
Ethical approval was not taken for this study as for this study no patient was visited at any point of time and it only involved the samples received in Bacteriology lab for routine processing. No identifier is used in this study.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Laboratory diagnosis of lower respiratory tract infections: Controversy and conundrums. J. Clin. Microbiol.. 2002;40:3115-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Composition and immunological significance of the upper respiratory tract microbiota. FEBS Lett.. 2016;590:3705-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Multidrug-resistant bacteria in the community: An update. Infect. Dis. Clin. North Am.. 2020;34:709-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparison of six different criteria for judging the acceptability of sputum specimens. J. Clin. Microbiol.. 1982;16:627-31.
- [CrossRef] [PubMed] [Google Scholar]
- Microscopic and bacteriologic analysis of expectorated sputum. Mayo Clin Proc. 1975;50:339-44.
- [PubMed] [Google Scholar]
- Bacterial sputum cultures. A clinician’s viewpoint. Mayo Clin Proc.. 1977;52:39-41.
- [PubMed] [Google Scholar]
- Community-acquired pneumonia in adults: Guidelines for management the infectious diseases society of America. Clin. Infect. Dis.. 1998;26:811-38.
- [CrossRef] [PubMed] [Google Scholar]
- Infections of the Lower Respiratory Tract. In: Betty AF, Daniel FS, Alice SW, eds. Bailey and Scott’s Diagnostic Microbiology. Mosby; 2002. p. :884-98.
- [Google Scholar]
- Clinical microbiology procedures handbook (3rd edition). Washington, DC: ASM Press; 2010.
- The quality of sputum specimens as a predictor of isolated bacteria from patients with lower respiratory tract infections at a tertiary referral hospital, denpasar, bali-Indonesia. Front Med (Lausanne). 2019;6:64.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sputum quality assessment regarding sputum culture for diagnosing lower respiratory tract infections in children. Open Access Maced J Med Sci. 2019;7:1926-30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Guidelines for collection, transport, processing, analysis and reporting of cultures from specific specimen sources. In: Koneman’s colour atlas and textbook of Microbiology (6th edition). Lippincott: Williams and Wilkins publications; 2006. p. :68-111.
- [Google Scholar]
- A study on analysis of the sputum gram staining and culture in patients with lower respiratory tract infections attending a tertiary care hospital. Ind J Microb Res. 2016;3:24.
- [CrossRef] [Google Scholar]
- Diagnostic value of sputum gram’s stain and sputum culture in lower respiratory tract infections in a tertiary care hospital. Int. J. Curr. Microbiol. App. Sci. 2017;6:4310-4.
- [Google Scholar]
- Implementation of Routine Sputum Rejection Criteria to Improve the Outcome of Sputum Culture Results. Int Medical J. 2008;15:287.
- [Google Scholar]






