Translate this page into:
A rare incidence of malignant pleural effusion in multiple myeloma
*Corresponding author: Dr. Kundan Mishra, Department of Hematology, Army Hospital (Research & Referral), Delhi, India. mishrak20@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Boddu R, Singh K, Saxena P, Pattanayak S, Kumar S, Mishra K, A rare incidence of malignant pleural effusion in multiple myeloma. Ann Natl Acad Med Sci (India) 2025;61:27-30. doi: 10.25259/ANAMS-2023-9-3-(1025)
Abstract
Multiple myeloma (MM) is one of the most common hematological malignancies. The incidence of pleural effusion (6%) as such is rare in patients with MM, and the incidence of myelomatous pleural effusion is even rarer (1%). The timely diagnosis of this entity is very important because these individuals have a poor overall prognosis. Diagnosis can be made by careful examination of pleural fluid cytology and can be confirmed by flow cytometry. We report a case of myelomatous pleural effusion diagnosed early and successfully managed.
Keywords
Extra-medullary disease
Malignant pleural effusion
MM
Multiple myeloma
Myeloma
INTRODUCTION
Multiple myeloma (MM), a hematological malignancy, is characterized by clonal plasma cells. These cells typically involve the bone marrow found in flat bones (skull, ribs, pelvis, and vertebrae), and the clonal immunoglobulins infiltrate the soft tissues (kidney, nerves, and lungs).1,2 It is one of the most common hematologic malignancies and constitutes about 1% of all malignancies. However, pleural effusion in patients with MM is rarely reported in the literature with an incidence of about 6%; moreover, malignant myelomatous pleural effusion (MPE) occurs in less than 1% of patients.3 Sometimes, this may be the first clinical manifestation of myeloma and may also develop during the course of treatment in a few patients. In the last two decades, only about 20 cases of MM with malignant pleural effusion have been published in English literature. We herein report a case of MPE, treated successfully with velcade, pomalidomide, and dexamethasone (VPd) regimen and radiotherapy.
CASE REPORT
We present a 55-year-old male with MM (immunoglobulin G [IgG] kappa, negative myeloma fluorescence in situ hybridization [FISH] panel), who received six cycles of VPd regimen. After completion of six cycles of VPd, he was asymptomatic, discontinued his therapy, and lost to follow-up. He again presented to us with complaints of worsening breathlessness and easily fatigued. He also gave a history of 4 kg weight loss over the last two months. On clinical examination, he was pale and had tachypnea (respiratory rate 28/minute). On chest auscultation, the air entry was decreased on the left side (infrascapular and infra-axillary areas). His blood counts showed hemoglobin (Hb) 10.7g/dL, white blood cells (WBCs) 5400/µL, and platelets 185,000/µL. His bilirubin, aspartate transaminase (AST), and alanine transaminase (ALT) were normal, but kidney function tests revealed azotemia (creatinine 1.56 mg/dL). Serum protein electrophoresis showed an M spike of 3.2 g/dL, IgG kappa band on immunofixation, and free light chain assay showed κ-2384, λ-6.35 with a ratio of 375.4. Chest X-ray posteroanterior (PA) view image showed left-sided cerebellopontine angle (CPA) fullness with a meniscus, suggestive of pleural effusion [Figure 1]. Computed tomography images of the chest showed pulmonary pathology confined to the left side of the lung in the form of loculated pleural effusion with underlying lung collapse. There were also fluids in the fissures [Figure 2a–2c]. The pleural fluid (PF) was straw-colored and biochemical analysis of the PF showed protein 5.5 g/dL (serum protein 6.2 g/dL) and lactate dehydrogenase (LDH) 396 U/L (serum LDH 350 U/L), pathognomic of exudative pleural effusion. Cytological study of the PF showed WBC of 800 cells/μL, with a predominance of lymphocytes. PF Zeihl-Neilsen stain and geneXpert for tuberculosis were negative, adenosine deaminase was raised to more than ten times the upper limit of the normal (33 U/L, normal range <30 U/L). PF cultures were sterile. A clinical diagnosis of pleural effusion, likely of tubercular origin, was made, and the patient was treated with four drugs (isoniazid, rifampicin, pyrazinamide, and ethambutol) anti-tubercular therapy (ATT). As the pleural effusion was loculated, therapeutic pleurocentesis was done, followed by pigtail insertion. Subsequent PF cytology showed WBC of 1200 cells/μL with numerous immature plasma cells (bilobed and multilobed plasma cells) [Figure 3a and 3b]. A flow cytometry of the PF showed a predominance of CD38/CD138 positive cells (58% of all nucleated cells) and these cells also expressed positivity for CD56 and CD200 with kappa restriction [Figure 4a–4c].
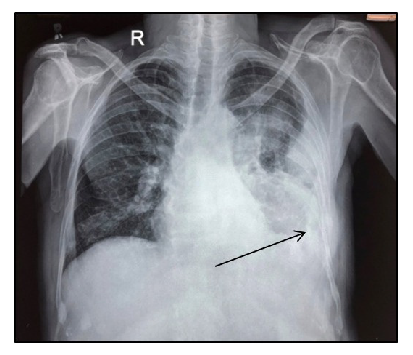
- Chest-X-ray showing massive pleural effusion with blunting of costophrenic angle on the left side (black arrow).
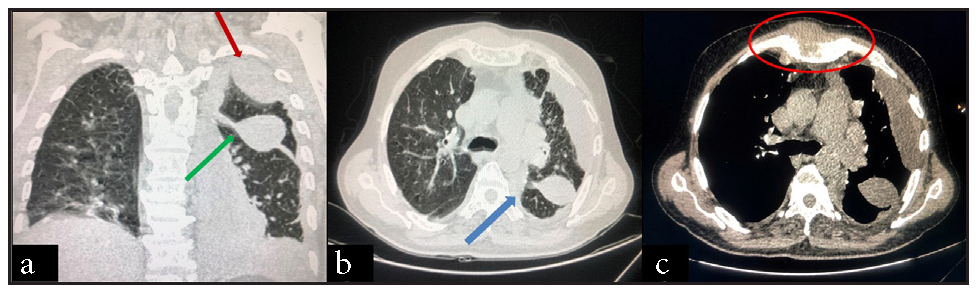
- CT chest images showing (a) loculated pleural effusion (green arrow) with fissural effusion (red arrow), (b) with collapse consolidation of underlying lung on the left side (blue arrow). (c) Multiple lytic lesions can be seen in the visualized bones and soft tissue component of the plasmacytoma on the sternum and left first rib (red circle). CT: Computed tomography.
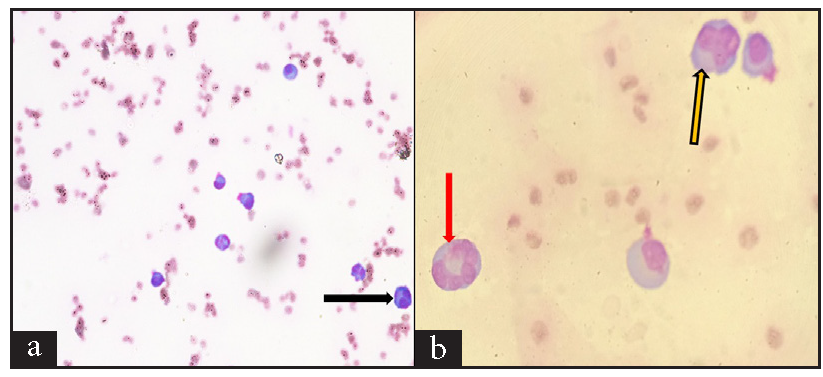
- Wright-Geimsa stained smears show mature and immature plasma cells, including (a) 400X, Bilobed plasma cell (black arrow), and (b) 1000X, multilobed plasma cells (red and yellow arrows).
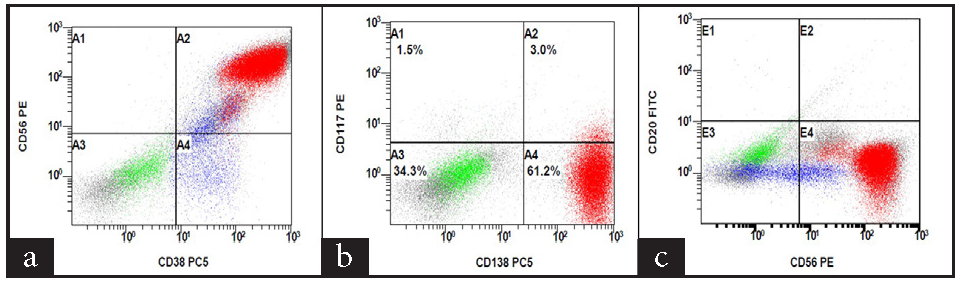
- Flow cytometry scatter plots showing (a) CD38, (b) CD138, (c) CD56 positivity.
A final diagnosis of “malignant pleural effusion in a patient with multiple myeloma” was made. His ATT was stopped and restarted on the VPd regimen. He also received radiotherapy over bony lesions. After two weeks, he was subjectively better, with improvement in pain and dyspnea. After four weeks of follow-up, he had complete relief from dyspnea and a repeat X-ray of the chest showed clearing of pleural effusion.
DISCUSSION
MM presents with a variety of symptoms. Though bone pain and anemia are the most common presentation, renal failure, hyperviscosity-related features, and symptoms due to associated amyloidosis are often seen.4,5. Lung involvement, especially the effusion, is rarely seen in MM the estimated incidence of effusion is around 5–6% and is mainly non-myelomatous. The occurrence of MPE is even rarer and seen in less than 1% of all cases.3 In developing countries, tuberculosis is the most common cause of pleural effusion.6 Other common etiologies of pleural effusion in patients with MM are congestive heart failure, cirrhosis, kidney disease, pulmonary infarcts (pulmonary thromboembolism), hypoalbuminemia, and rarely myelomatous effusion. The proposed pathogenetic mechanisms of MPE include direct extension from chest wall plasmacytomas, invasion from adjacent skeletal lesions, and infiltration of pleura by myeloma.7 Left-sided effusion is more common and bilateral effusion is seen in only three cases so far in the published literature.8 However, it is not clear whether this is due to any underlying genetic mutations. The appearance of immature plasma cells is very characteristic on a cytology smear and is considered the best diagnostic technique.9 Moreover, specific stains, particularly the May-Grunwald-Giemsa (MGG) and Periodic Acid-Schiff (PAS), can also be used to highlight these plasma cells. Whole body positron emission tomography-computed tomography (WB PET-CT) scan can confirm the involvement of the pleura, but it cannot definitively determine the existence of MPE. Flow cytometry is a rapid, accurate, and objective analytic method in diagnosing MPE than conventional cytology. It is imperative to know about this rare entity and needs exclusion from other common secondary causes of pleural effusion like tuberculosis in patients with MM. MPE typically develops as a late complication of the disease and is known to be a poor prognostic indicator. Previous studies have reported that patients with MPE have a median survival time of less than four months.10 However, newer drugs like pomalidomide, carfilzomib, and daratumumab are more active in MM and have brought new hopes for myeloma patients, though newer drugs bring newer challenges in the form of associated adverse events.11–14 A summary of previously published articles have been summarized in Table 1.15–19 The index patient was treated with pomalidomide-containing regimen (VPd) and showed a good response. However, further follow-ups will be required to assess the survival benefit of newer drugs in MM with pleural effusion.
| Author | No. of Pts | Presenting feature | Age/Sex | Disease status at MPE | Unilateral or bilateral | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Iqbal N et al.15 (2016) |
N = 1 Case report |
Pleural effusion Renal failure |
68/M | Inaugural | Unilateral | VRD |
Excellent response Well at one year |
| Ghorbel IB et al.16 (2015) |
N = 5 Case series |
Bony pains | 74/M | Relapse | Bilateral | MP | LTF |
| Dull right chest pain | 62/M | Inaugural | Unilateral | VAD | Died few months later | ||
| Bony pains | 62/F | Relapse | Unilateral | - | Died in few days | ||
| Dyspnoea and chest pain | 52/M | Inaugural | Unilateral | VAD | Died at four months | ||
| Dyspnoea and low back ache | 55/M | Inaugural | Unilateral | MP | Died few months later | ||
| Miller J et al. 17 (2012) |
N=1 Case report |
Dyspnoea | 66/F | Relapse | Unilateral | VCD à MP | No follow-up available |
| Maat Z et al. 18 (2022) |
N = 1 Case report |
Cough and chest pain | 44/M | Inaugural | Unilateral | VRD | Died in few days (meningitis) |
| Wada A et al. 19 (2020) |
N = 1 Case report |
50/F | Relapse | Unilateral | VRD à DaraRD | Two years RFS |
MPE: Myelomatous Pleural Effusion, VRD: Bortezomib, Lenalidomide, Dexamethasone, MP: Melphalan Prednisolone, LTF: Lost to follow up, VAD: Bortezomib, Doxorubicin, Dexamethasone, VCD: Bortezomib, Cyclophosphamide, Dexamethasone, RFS: Relapse free survival, DaraRD: Daratumumab, Lenalidomide, Dexamethasone
CONCLUSION
Though MPE is rare, clinicians should not be complacent while investigating MM patients with pulmonary findings suggestive of MPE. Repeated sampling and careful examination of pleural cytology and flow cytometry are crucial in the diagnosis. Though the presence of MPE suggests poor prognosis, an early diagnosis and prompt initiation of anti-myeloma therapy can indeed be the right way for a successful outcome, as in the index case.
Authors’ contributions
RB, KS, PS, SP, SK and KM: Were involved in patient management. The manuscript was written by RB and it was vetted by all the authors.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Priapism as the presenting manifestation of multiple myeloma. Indian J Hematol Blood Transfus. 2017;33:133-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Thoracic and pulmonary abnormalities in multiple myeloma. A review of 958 cases. Arch Intern Med. 1978;138:727-30.
- [PubMed] [Google Scholar]
- Hyperviscosity syndrome complicating immunoglobulin G myeloma: Cognisance of early plasmapheresis is crucial. Semin Dial. 2023;36:175-7.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculous pleural effusions: Advances and controversies. J Thorac Dis. 2015;7:981-91.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Myelomatous Pleural Effusion: Case report and review of the literature. Sultan Qaboos Univ Med J. 2011;11:259-64.
- [PubMed] [PubMed Central] [Google Scholar]
- Myelomatous pleural effusion as an initial sign of multiple myeloma-a case report and review of literature. J Thorac Dis. 2014;6:E152-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pleural effusion and multiple myeloma – more than meets the eye: A case report. Mol Clin Oncol. 2021;15:238.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical characteristics and prognosis of multiple myeloma with myelomatous pleural effusion: A retrospective single-center study. Technol Cancer Res Treat. 2022;21:15330338221132370.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Real world experience with “generic” pomalidomide in relapsed refractory multiple myeloma. Leuk Lymphoma. 2019;60:1102-4.
- [CrossRef] [PubMed] [Google Scholar]
- Role of daratumumab in the frontline management of multiple myeloma: A narrative review. Expert Rev Hematol. 2023;16:743-60.
- [CrossRef] [PubMed] [Google Scholar]
- Poor mobilisation after daratumumab based combination chemotherapy in patients of newly diagnosed multiple myeloma. Indian J Hematol Blood Transfus. 2019;35:584-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sudden cardiac arrest after daratumumab infusion. Indian J Med Paediatr Oncol. 2019;40:301-3.
- [CrossRef] [Google Scholar]
- Pleural effusion as a manifestation of multiple myeloma. BMJ Case Rep. 2016;2016:bcr2016215433.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pleural myelomatous involvement in multiple myeloma: Five cases. Ann Saudi Med. 2015;35:327-30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Myelomatous pleural effusion: A case report. Respir Med Case Rep. 2012;5:59-61.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Myelomatous pleural effusion: A rare case report and literature review. Case Rep Oncol. 2022;15:1049-54.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Successful treatment of myelomatous pleural effusion with daratumumab administration before autologous peripheral stem cell transplantation. Rinsho Ketsueki. 2020;61:879-84.
- [CrossRef] [PubMed] [Google Scholar]





