Translate this page into:
A Study on the Impact of Diabetes Mellitus on the Severity of COVID-19-Associated Mucormycosis
Address for correspondence Siddharth Madan, MS, DNB, FICO, FAICO (Retina), MNAMS, Department of Ophthalmology, University College of Medical Sciences and Associated GTB Hospital, India (e-mail: drsiddharthmadan@gmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
Diabetes mellitus (DM) seems the most common predisposing factor for rhino-orbito-cerebral mucormycosis (ROCM). This study aimed to study the impact of DM on the severity of COVID-19-associated ROCM (CAM).
Methods:
This was a retrospective analytical study performed over a period of 3 months to assess the impact of DM on the severity of CAM in 100 patients and association of clinical correlates of DM with severity of CAM.
Statistical analysis:
The data collected using the study tools were converted into a computer-based spreadsheet and analyzed. The statistical analysis comprised a descriptive analysis that involved calculating means, standard deviations, and proportions. For calculating the significance of the difference of mean between two groups, Student's t-test was applied. In addition, chi-square test (or Fisher's t-test if applicable) was applied to study the significance of association of clinical correlates of DM with severity of CAM for categorical variables and t-test for continuous variables.
Results:
The prevalence of DM was 67%. The average presenting blood sugar level was 245.9 ± 99.86 mg%. Glycated hemoglobin level between 4.5 and 6.5% was observed in 57 patients and over 6.5% in 43 subjects. A high body mass index (BMI) of 25 and above was noted in 52 patients. A significantly higher level of presenting blood sugar and a longer duration of hospital stay was noted in patients having stage 3b or higher (p < 0.05) when compared with those having stage 3a or below. No significant correlation was observed in patients in stage 3a or below and those presenting with stage 3b or higher in terms of BMI, waist to hip ratio, or total cholesterol levels. There was a strong correlation between blood sugar level at presentation, severity of DM with the severity of ROCM, and a strong inverse correlation noted between HDL level and severity of ROCM.
Conclusion:
A poor metabolic control is associated with a higher risk of a severe disease with intracranial involvement.
Keywords
ROCM
COVID-19
orbital mucormycosis
diabetes mellitus
corticosteroids
Introduction
A recent increase in the number of cases of rhino-orbito-cerebral mucormycosis (ROCM) amidst the COVID-19 pandemic is a matter of concern. Most cases were observed in patients suffering from diabetes mellitus (DM) especially in the ones who had poor metabolic control of diabetes and/or had severe underlying COVID-19.1 Mucormycosis is a potentially fatal disease characterized by vascular invasion due to saprophytic fungi that belong to the order Mucorales. The fungus is ubiquitously present in the environment, flourishes in the Indian soils, and therefore India has the highest number of cases of mucormycosis in the world with a prevalence of 0.14 cases per 1000 population.2
Rhizopus arrhizus (most common), Rhizopus microspores, and Rhizopus homothallicus are among the common species responsible for causing mucormycosis in India and the rest of the world.3 The commonest type of mucormycosis observed in India include ROCM (45 − 74%), followed by cutaneous form (10 − 31%), pulmonary (3 − 22%), gastrointestinal (2 − 8%), disseminated and renal type (0.5 − 9%).3 However, rhino-orbital (ROM) and ROCM account for the majority of cases (89%) of mucormycosis presenting in India amidst the COVID-19 pandemic. Pulmonary and disseminated types are also observed (10%).4,5
A recent study by John et al reported a series of ROM in patients who were documented to be nondiabetic prior to contracting COVID-19 and later diagnosed with diabetes mellitus of recent onset. Diabetes mellitus was uncontrolled in about 67% of the patients and 94% of the patients had a mean glycated hemoglobin (HbA1c) of 10% reflecting a poor control of DM. Mucormycosis is aptly addressed as diabetes-defining illness. Recently, it was reported that about 77% cases of ROCM are observed in patients diagnosed with diabetes mellitus.3 Data from 18 countries reiterated the fact that diabetes is the predominant risk factor (95.2%) responsible for causing COVID-19 associated rhino-orbito-cerebral mucormycosis (CAM) cases in India, with uncontrolled or poorly controlled diabetes (80.3%) being the commonest presentation and diabetic ketoacidosis (DKA) was observed in 41% of cases.6 A systematic review of 101 cases of CAM further strengthened the observation that the majority (81%) of the cases were from India and 83% of the subjects had underlying hyperglycemia at presentation. Diabetic ketoacidosis was seen in 15% of the cases. Also, 76.3% of the patients received corticosteroid therapy for treatment of COVID-19.7
Materials and Methods
This study aimed to evaluate the impact of DM and its clinical correlates on the severity of CAM. The primary objective of the research was to assess the prevalence of DM in patients with CAM. The secondary objective was to assess the impact of diabetes mellitus on the severity of CAM and determine the association of clinical correlates of DM with the severity of CAM. The study was conducted in the Department of Ophthalmology, Department of Medicine, and Department of Otorhinolaryngology at a tertiary care center in Delhi. The records of the patients who got admitted between August 2021 and October 2021 were analyzed. This was a retrospective, analytical study. Institutional Ethics Committee–Human Research (IEC-HR) approval was taken and subject confidentiality and anonymity was maintained in all cases. The study was conducted according to the principles of the Declaration of Helsinki. There are no conflicts of interest or financial disclosures. The patients included in the study comprised confirmed cases/suspected cases of COVID-19 who presented with either clinical, radiological, microbiological, cytopathological, or histopathological evidence suggestive of mucormycosis. The patients excluded from the study were those with no clinical, radiological, microbiological, cytopathological/histopathological evidence suggestive of ROM; patients with HIV-positive status; patients on any form of immunosuppression including cancer chemotherapy and those under the age of 18 years.
All hospital records of subjects fulfilling the inclusion criteria over a time period between August 2021 and October 2021 were retrieved and studied. Hospital records with only complete information on the variables of interest of the current study, with no missing data, were analyzed eventually. There was no sampling involved. The number of such case records during the stipulated period was approximately 100. The patients were placed in two groups based on the severity of mucormycosis for the purpose of statistical analysis. The groups were divided into stage 3a and below and those with stage 3b (diffuse orbital involvement) and/or higher. Further patients with nasal and/or sinus involvement (Stages 1 and 2 as per classification—less severe variety) were compared with those presenting with orbital and/or intracranial extension (Stages 3 and 4), being the more severe variety. The diagnosis of DM was decided on the basis of patient history or the HbA1c values. The diagnosis of Diabetes was done as per American Diabetes Association (ADA) guidelines- Fasting blood sugar (FBS) ≥ 126 mg/dL, 2 hours post prandial blood sugar (PPBS) ≥ 200 mg/dL or HbA1c ≥ 6.5%.8 The recent onset diabetes mellitus was defined as FBS ≥ 126 mg/dL or 2 hours PPBS ≥ 200 mg/dL, which was diagnosed first time during the present illness with a negative history of diabetes or a history of normal glycated hemoglobin level and no history of steroid use.9 The steroid induced diabetes mellitus was diagnosed as FBS ≥ 126 mg/dL or 2 hours PPBS ≥ 200 mg/dL diagnosed first time during the present illness with the history of use of steroids with no prior history of diabetes or raised glycated hemoglobin levels.10
Clinical history of the cases including history of COVID-19, comorbidities were obtained as per records. General physical examination including blood pressure, measurement of body mass index (BMI), waist circumference as clinical correlates of diabetes were tabulated based on the records. Reports of the routine biochemical test including glycemic control indices (fasting blood sugar, postprandial blood sugar, HbA1c), serum lipid profile, and HIV status of the cases was retrieved from records. The severity of mucormycosis was staged based on the classification proposed by Honavar et al.11
Stage 1 (1a, 1b, 1c) had involvement of the nasal mucosa
Stage 2 (2a, 2b, 2c, 2d) had involvement of the paranasal sinuses
Stage 3 had orbital involvement:
Stage 3a- Nasolacrimal duct, medial orbit, vision unaffected; 3b-diffuse orbital involvement, vision unaffected; Stage 3c-central retinal or ophthalmic artery occlusion or superior ophthalmic vein thrombosis, involvement of the superior or inferior orbital fissure, orbital apex with loss of vision; Stage 3d-bilateral orbital involvement
Stage 4-had involvement of the central nervous system (CNS) with the disease.
The outcome measures of the study were ascertained based on the difference in severity grading of CAM at presentation among patients with DM and those without diabetes. Further the hospitalization duration, requirement of critical care, surgical intervention, and mortality between the patients with DM was studied.
Statistical analysis: The data that were collected using the study tools were converted into a computer-based spreadsheet and analyzed. The statistical analysis comprised a descriptive analysis that involved calculating means, standard deviations, and proportions. For calculating the significance of difference in the mean HbA1c level between the groups based on the severity of CAM, Student's t-test was applied. In addition, chi-square test (or Fisher's t-test if applicable) was applied to study the significance of association of clinical correlates of DM with severity of CAM for categorical variables and t-test for continuous variables.
Results
The prevalence of diagnosed DM was 67% in this study, ignoring the recent onset and the steroid induced DM (n = 11). All diagnosed DM patients were of type 2 DM and the average duration of diabetes was 3.78 years (30.4 days–24 years). Twenty-two subjects were not diabetic. Out of a total of 100 patients (male-61, female-39) included in the study population with an average age of 52.1 ± 11.29 years (25 to 78 years), 31 were aged 60 years or above (geriatric population) and 69 were under the age of 60 years. ►Table 1 shows the demographic profile of the patients. Stage wise distribution was performed based on the staging criteria in 100 patients (stage 2-18, stage 3a-25, stage 3b-19, stage 3c-23, stage 4-15). Positive history for COVID-19 or positive testing on RTPCR/RAT was observed in 49 patients. Thirty-five patients had a history of steroid consumption for COVID-19. Sixty-four patients were receiving oral hypoglycemic medications, three were on insulin and six were on combination therapy of both oral medications and insulin for control of the blood sugar levels. Recently diagnosed DM or DM as a result of steroid administration was noted in three (27.3%) and eight (72.7%) patients respectively. The average presenting blood sugar level was 245.9 ± 99.86 mg% (100–578 mg%). Glycated hemoglobin level between 4.5 and 6.5% was observed in 57 patients and over 6.5% in 43 subjects. The average HbA1c level in 100 patients was 6.5 ± 1.37% (4.6–12.3%). ►Figure 1 shows the correlation between the severity of diabetes as measured by the HbA1c level and the severity of ROCM as measured by the stage. Body mass index (BMI) of 25 and above was considered as being overweight that got observed in 52 patients. Total cholesterol, high density lipids and serum triglycerides were raised in 2, 100, and 38 patients, respectively. Based on the World Health Organization (WHO) criteria, a waist to hip ratio of over 102 in men and over 88 in women was considered abnormal.12
| Parameter n = 100 | Observation |
|---|---|
| Geriatric | Yes–31, No-69 |
| Gender | Male-61, females-39 |
| Stage of ROCM | Stage 2-18 Stage 3a-25 Stage 3b-19 Stage 3c-23 Stage 4-15 |
| Diabetes mellitus (DM) | Diagnosed DM- 67/100 (67%); Recent-onset and steroid induced- 11; No DM -22 |
| Comorbidities | |
| Hypertension | No- 87, Yes- 13 |
| Coronary artery disease; Hypothyroidism; Past tuberculosis; Hepatitis B; HIV |
Yes- 4, No- 96; Yes-3, No-97; Yes-2, No-98; Yes-1, No-99; HIV positive -1 |
| Positive history for COVID-19/currently positive | Yes-49, No-51 |
| History of steroid intake | Yes-35, No-65 |
| Type of steroid intake | Oral-9 Intravenous-19 Both Oral/IV-7 |
| Antidiabetic medication | Oral-64 Insulin-3 Oral and insulin-6 |
| Insulin requirement during hospitalization | Yes-83, No-17 |
| HbA1c | Level 4.5-6.5%- 57 Level > 6.5%- 43 |
| Body mass index (BMI) | < 25-48/100 (48%) ≥ 25-52/100 (52%) |
| Cholesterol > 200 High density lipid (HDL)-men > 40, female > 50 Triglyceride (TG) level >150 |
Total cholesterol Elevated-23 Normal-77 HDL Low levels-100 TG Elevated-38, Normal-62 |
| Waist to hip ratio (WHO criteria) men >102, Female > 88; for Asians (men > 90, female > 80) | Following WHO criteria- Increased–53 Not increased- 47 Following Asian criteria Increased-98 Not increased-2 |
| Treatment | |
| Status of treatment | Critical care-16 Routine management without critical care-84 Discharged-97 Death-3 |
| No operative procedure Endoscopic sinonasal debridement (SND) External SND/maxillectomy Exenteration Neurosurgical procedure |
No operative procedure-2 No surgery done as was unfit for Surgery-1 Endoscopic sinonasal debridement (SND)-19 Endoscopic and external SND-2 External SND/maxillectomy-59 External SND and exenteration-16 |
| Exenteration Done | Yes-17, No-83 |
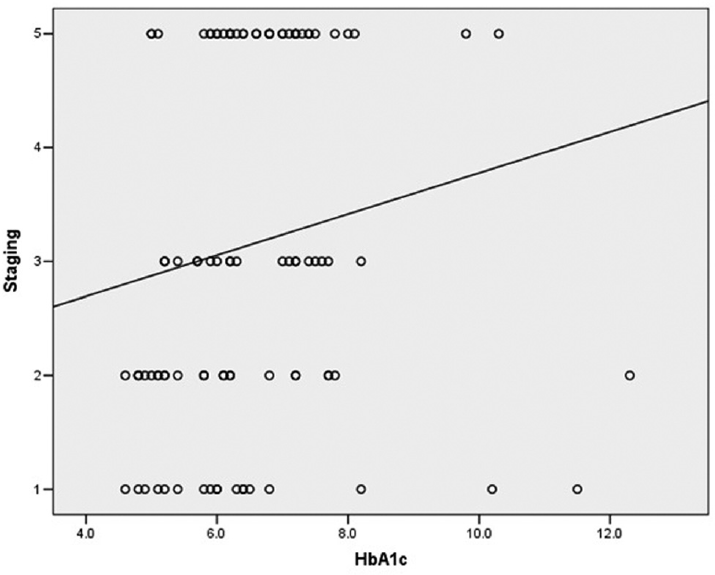
- Correlation between severity of diabetes (as measured by the HbA1c level) and severity of Rhino-orbital mucormycosis (as measured by the stage).
For Asians, a level of (men >90, female> 80) was considered as abnormal. This was raised in 53 and 98 patients based on the WHO and Asian criteria, respectively. Ninety-six patients underwent sinonasal debridement (external and/or endoscopic) and 17 were subjected to orbital exenteration. ►Table 2 highlights the mean value for various parameters that were studied. ►Table 2 also gives the correlation between severity of diabetes and severity of rhino-orbital mucormycosis and association of severity of ROM with other cofactors. Various parameters were compared for patients having stage 3a and below, i. e., stage 2 when compared with stage 3b and higher. A significantly higher level of presenting blood sugar levels (value = 0.017) and a longer duration of hospital stay (p = 0.00) was noted in patients having stage 3b or higher (p < 0.05) when compared with those having stage 3a or below. The patients with stage 3b, 3c, or 4 had a significantly lower levels of high-density lipoprotein when compared with the patients having stage 3a or below (p = 0.020). A significantly higher number of patients (n = 50 [61.7%]) who had DM had presentation with stage 3b or above (p = 0.049). Further the patients who had diagnosed DM [n = 42 (62.7%)] presented with stage 3b or above (p = 0.021). A significantly (p = 0.005) higher level of presenting blood sugar over 200 mg% was noted in patients (n = 40 [69.0%]) who presented with stage 3b or higher as were significantly higher levels (p = 0.008) of HbA1c over 6.5% (n = 31 [72.1%]) at presentation (►Table 3). No significant correlation was observed in patients in stage 3a or below and those presenting with stage 3b or higher in terms of BMI, waist to hip ratio, or total cholesterol levels. 53.5% of those with high HbA1c (≥ 6.5%) were overweight (as per the WHO classification) or obese (as per recommended classification for Asian Indians, JAPI, Misra et al, 2009).12 Also, 60.5% had central obesity, by waist circumference (by the WHO cutoffs of 102 cm for males and 88 cm for females) (98.0% if we take as per the recommended classification for Asian Indians, JAPI, Misra et al, 2009). Those with higher HbA1c were significantly more likely to have Stage > 3a (3b or higher) (72.1%) as compared to those with normal HbA1c (45.6%), p-value for difference = 0.008. Two deaths were reported as outcome among the 57 patients with elevated HbA1c (≥ 6.5%), as compared to one death among the 43 patients with normal HbA1c. Regression analysis was performed adjusting for confounding effect of BMI and gender and it was observed that HbA1c remained a strong correlate with severity of DM.
| Mean | Std. deviation | Minimum | Maximum | |
|---|---|---|---|---|
| Various parameters | ||||
| Age (n = 100) | 52.1 | 11.29 | 25 | 78 |
| Interval between COVID-19 and mucormycosis for those who had known history of COVID-19 (n = 48) | 28.0 | 15.48 | 3 | 65 |
| Hospital stay for COVID-19 for those who had some hospital stay (n = 25) | 15.6 | 10.66 | 5 | 45 |
| Blood sugar at presentation | 245.9 | 99.86 | 100 | 578 |
| HbA1c | 6.5 | 1.37 | 4.6 | 12.3 |
| Total hospital stay | 66.7 | 14.68 | 42 | 102 |
| BMI | 25.6 | 4.40 | 16.81 | 38.14 |
| TG | 142.8 | 47.57 | 52 | 315 |
| HDL | 28.1 | 5.50 | 19 | 45 |
| Total cholesterol | 166.0 | 44.52 | 62 | 280 |
| Association between different variables and stage of mucor {0- Stage 2, 3a; 1- Stage 3b, 3c, 4) | |||||
|---|---|---|---|---|---|
| Variable | Type of variable | Stage 2,3a | Stage 3b, 3c, 4 | p-Value | |
| Geriatric age (y) | Age < 60 Age ≥ 60 |
31 (44.9%) 12 (38.7%) |
38 (55.1%) 19 (61.3%) |
0.561 | |
| Gender | Female Male |
16 (41.0%) 27 (44.3%) |
23 (59.0%) 34 (55.7%) |
0.750 | |
| Diabetes mellitus | No DM DM present |
12 (63.2%) 31 (38.3%) |
7 (36.8%) 50 (61.7%) |
0.049 | |
| Hypertension | No Yes |
39 (44.8%) 22 (44.9%) |
30 (58.7%) 27 (55.1%) |
0.340 | |
| Positive history for COVID-19 | No Yes |
21 (41.2%) 62 (63.9%) |
Yes- 75 (64.1%) Yes- 35 (36.1%) |
0.707 | |
| Whether received steroids | No Yes |
26 (40.0%) 17 (48.6%) |
39 (60.0%) 18 (51.4%) |
0.409 | |
| Diabetes mellitus | No DM Recent or Steroid induced |
15 (68.2%) 3 (27.3%) |
17 (31.8%) 8 (72.7%) |
0.021 | |
| Diagnosed DM | 25 (37.3%) | 42 (62.7%) | |||
| Blood sugar at presentation (Random blood sugar > 200 mg %) | No Yes |
25 (59.5%) 18 (31.0%) |
17 (40.5%) 40 (69.0%) |
0.005 | |
| HbA1c | 4.5-6.5% >6.5% |
31 (54.4%) 12 (27.9%) |
26 (45.6%) 31 (72.1%) |
0.008 | |
| BMI | < 25 ≥25 |
18 (37.5%) 25 (48.1%) |
30 (62.5%) 27 (51.9%) |
0.286 | |
| Waist to hip ratio Men >102, Female> 88 |
Normal High |
19 (40.4%) 24 (45.3%) |
28 (59.6%) 29 (54.7%) |
0.624 | |
| Total cholesterol | <200 >200 |
33 (42.9%) 10 (43.5%) |
44 (57.1%) 13 (56.5%) |
0.958 | |
| Staging {Stage 3a and below (0) when compared with Stage 3b and higher (1)} | N | Mean | Std. deviation | p -Value | |
| Blood sugar at presentation | 0 | 43 | 218.67 | 98.976 | 0.017 |
| 1 | 57 | 266.53 | 96.338 | ||
| HbA1c | 0 | 43 | 6.270 | 1.7013 | 0.101 |
| 1 | 57 | 6.725 | 1.0334 | ||
| Total hospital Stay | 0 | 43 | 60.86 | 12.176 | 0.000 |
| 1 | 57 | 71.21 | 14.929 | ||
| BMI | 0 | 43 | 26.1342 | 4.29388 | 0.385 |
| 1 | 57 | 25.3553 | 4.50383 | ||
| HDL | 0 | 43 | 29.60 | 5.368 | 0.020 |
| 1 | 57 | 27.04 | 5.392 | ||
There was no statistically significant correlation between patients who underwent exenteration when compared with their age, gender, presence/absence of DM, positive history/currently positive status for COVID-19 on testing, HbA1c levels, or HIV status (p > 0.05). However, when subjects presenting with stage 2 and below were compared with stage 3 and 4, the patients with stage 3, 4 had a significantly higher BMI (p = 0.058), waist to hip ratio (p = 0.020), higher cholesterol level (p = 0.011) and almost significantly higher levels of presenting blood sugar (p = 0.070). As far as the secondary objectives of the study are concerned, there was a strong correlation between blood sugar level at presentation and the severity of ROM (Spearman's rho = 0.260, p = 0.009) and so was a strong inverse correlation noted between HDL level and the severity of ROM (Spearman's rho = −0.237, p = 0.018). All ROCM patients had low HDL levels. The correlation between severity of diabetes (as measured by the HbA1c level) and severity of rhino-orbital mucormycosis (as measured by the stage) was found to be statistically significant (Spearman's rho = 0.282, p = 0.004). The association was direct in nature, higher HbA1c was associated with a higher stage).
Discussion
It is now established that diabetes is associated with a poor prognosis of COVID-19.13,14 Several epidemiological studies have reported that patients suffering from diabetes mellitus are at a greater risk of getting hospitalized and also getting admitted to critical care alongwith a higher risk of mortality as a result of COVID-19.15,16,17 Mortality following the COVID-19 pandemic is noted to be 1.49 to 3 times higher in the individuals suffering from diabetes.18,19 It is also observed that individuals from black, Asian and minority ethnic (BAME) backgrounds are at a relatively higher risk of contracting SARS CoV-2 and the risk of death was also observed to be higher in the UK in those individuals suffering from type 1 and type 2 diabetes. This possibly could be a confounding factor in the regional differences and higher prevalence of CAM observed in India after the second wave of COVID-19.20,21,22,23 Newly diagnosed DM is commonly observed in COVID-19 patients as reported in the literature.24,25 A systematic review and meta-analysis of eight studies is reported with regard to the proportion of newly diagnosed diabetes in COVID-19 patients.26 Newly diagnosed diabetes in this study was defined as new-onset diabetes without any prior history of diabetes with fasting plasma glucose (FPG) ≥ 7.0 mmol/L or random blood glucose (RBG) ≥ 11.1 mmol/L and HbA1c < 6.5% or previously undiagnosed diabetes (FPG ≥ 7.0 mmol/L or RBG ≥ 11.1 mmol/L and HbA1c ≥ 6.5% or HbA1c ≥ 6.5% only.27 This is supported by reports showing exceptionally high insulin requirements in severely or critically ill COVID-19 patients with diabetes.24,28 There exists a complex mechanistic and clinical interplay between DM and COVID-19.
The management guidelines for the treatment of COVID-19 seem nonuniform in different centers/regions of the country. This is reflected in routine clinical practice where patients with different severity of COVID-19 receive intravenous and oral steroids where the same may not be warranted.29,30,31 Although dexamethasone gave a survival benefit by reducing mortality in the patients affected with COVID-19, the administration of corticosteroids remains a double-edged sword. This indiscriminate use of corticosteroids apart from other multiple factors has resulted in an epidemic of COVID-19-associated mucormycosis, specifically the cases of ROCM. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is no longer an exclusive pulmonary disease. It is shown to have involved the kidneys, brain, cardiovascular system (heart), eyes in the form of conjunctivitis, gastrointestinal and other endocrine organs.29,30,31
It is reported that the destruction of the pancreatic β-cell due to infection with the SARS CoV-2 causes dysregulation of the metabolism resulting in the diabetogenic effect of the virus.32 Involvement of the exocrine part of the pancreas presents as pancreatitis, enlargement of the pancreas, and alteration in the serum levels of amylase or lipase.33 COVID-19 also accounts for impairment of insulin receptor signaling along with a high degree of peripheral insulin resistance. All these disturbances in the physiology of the pancreas manifest in the form of elevated blood glucose levels in previously nondiabetic individuals who contract COVID-19. At 6 months after being infected with COVID-19, an increase in the burden of DM and use of oral hypoglycemic agents (OHA) is reported.34 Recently, studies are reported in previously non-diabetic individuals who contract COVID-19 presented with CAM and have now developed diabetes mellitus of recent onset.35,36,37 A global registry (COVIDiab) is set up to characterize the uncommon features of new-onset DM that are observed in COVID-19. In series of cases published on reviewing the literature, the average age of patients is reported to be from 35.9 to 60.5 years.37 The development of CAM in previously healthy patients who do not have underlying DM and are immunocompetent gives a pointer to the fact that there exist possibly other etiopathogenetic mechanisms that are responsible for this manifestation. Alterations in the mucosal immunity in the nasal cavity due to infection with SARS CoV-2 and loss of ciliary function causing a reduction in the nasociliary clearance might result in the proliferation of the fungal spores in the paranasal sinuses and nasal cavity, the primary sites that harbor spores of mucor.38,39 About 90.5% of patients suffering from CAM in Egypt had underlying diabetes on admission. In another study by Singh et al that had individuals mostly from India, about 80% had diabetes mellitus and 76.3% were administered corticosteroids.40 Dysregulation of the immune system, cytokine storm, coagulation in the microvasculature apart from thrombo-inflammation and immune exhaustion are noted as result of diabetogenic state due to COVID-19. Further, immunosuppression due to COVID-19 results in an altered function of the CD4+/CD8+ T cells and antigen-presenting dendritic cells. These factors are conducive for the proliferation of the fungus and development of ROCM.27,41,42 Platelet-driven immunosuppression is increasingly being recognized as a key factor in the etiopathogenesis of excessive COVID-19-related systemic inflammation that results in systemic co-infections apart from generalized immunosuppression.42,43
The existing literature on the research conducted on dyslipidemia and COVID-19 is limited; however, it is shown that dyslipidemia may play a role in the severity of COVID-19 infection.44 Future studies are needed. Further dyslipidemia in diabetes is observed in the form of high plasma triglyceride, low HDL cholesterol and increase in the concentration of small dense LDL cholesterol levels. A higher concentration of total cholesterol levels was noted in individuals with stage 3 and above and also was a higher mean HDL cholesterol level noted in patients with stage 3b and above. This could be a correlate of diabetes due to the associated dyslipidemia with the disease or could possibly be a result of COVID-19-associated dyslipidemia. Because the pre-COVID-19 lipid levels in these patients are not available with the authors, it seems uncertain to attribute this increase in the level of lipids to DM or COVID-19. However, it is certain that higher levels of total and HDL cholesterol were associated with a worse stage of ROCM at presentation.
High mortality amongst the affected individuals due to ROCM is known. About 60% mortality is reported despite aggressive treatment and 46% of the subjects have permanent blindness who survive.6 Surprisingly, the mortality rate due to CAM (36.5%) is lower when compared to the literature pertaining to the Indian population prior to the COVID-19 pandemic when the mortality reported was 52%.4 The lower mortality rate is attributable to the improvement in the diagnostic facilities and multidisciplinary management. Early sinonasal debridement is the key component of the management apart from prompt initiation of the systemic antifungal therapy, in the form of liposomal amphotericin B (10 mg/kg/day) intravenous for 6 weeks, constitutes the first-line treatment while the patient awaits surgery. If the patient is allergic to liposomal amphotericin B, intravenous/oral posaconazole (300 mg BID on day 1 followed by 300 mg/day for 6 months) is administered. Metabolic control primarily of the blood sugar levels is indispensable. Involvement of the CNS carries poor prognosis as reported in the literature.6
The study is novel as it highlights the occurrence of severe ROCM in patients who did not have a history of steroid consumption for management of COVID-19. Further, only one previous study exists that correlates serum lipid profile with the severity of CAM, wherein the authors concluded that a positive correlation exists between serum lipid profile and staging of ROCM and a negative correlation is observed between lipid levels with duration between onset of COVID-19 to onset of ROCM. This study concludes that a higher cholesterol level was noted in individuals presenting with a more severe stage of ROCM and a strong inverse correlation noted between HDL level and the severity of ROM wherein all ROCM patients had low HDL levels. Comparison of BMI and waist–hip ratio with severity of ROCM in this cohort of patients is also a new parameter that was evaluated. A large number of patients presenting to a single tertiary care center is also a key highlight of this research.
The results of the study show a high prevalence of DM in patients infected with mucormycosis who develop this fatal disease in the background of COVID-19. A spurt in the number of cases of ROCM to epidemic proportions was observed by the authors at the tertiary care center catering to over 300 patients who presented amidst the COVID-19 pandemic. The study was, however, limited by the fact that complete data for all patients was not available in the medical records due to the retrospective nature of the study and hence could not be evaluated completely.
Conclusions
A high prevalence of DM observed in this study reiterates that ROCM is a diabetes-defining disease and the incidence of ROCM increases in patients infected with SARS CoV-2. A poor metabolic control is associated with a higher risk of intracranial involvement. Prompt metabolic control with medical management with antifungal medications along-with sinonasal debridement with or without orbital exenteration is indispensable in reducing the mortality of the disease.
Conflict of Interest
None declared.
Funding
None.
References
- When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi (Basel). 2021;7(04):298. DOI: 10.3390/jof7040298
- [Google Scholar]
- Epidemiology of mucormycosis in India. Microorganisms. 2021;9(03):523. Published 2021 Mar 4. DOI: 10.3390/microorganisms9030523
- [CrossRef] [PubMed] [Google Scholar]
- A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect. 2020;26(07):944.e9-944.e15. DOI: 10.1016/j.cmi.2019.11.021
- [Google Scholar]
- Epidemiology and pathophysiology of COVID-19-associated mucormycosis: India versus the rest of the world. Mycopathologia. 2021;186(06):739-754. DOI: 10.1007/s11046-021-00584-8
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19-associated mucormycosis: an updated systematic review of literature. Mycoses. 2021;64(12):1452-1459. DOI: 10.1111/myc.13338
- [Google Scholar]
- The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancer Microbe. 2022;3(07):e543-e552.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15(04):102146. DOI: 10.1016/j.dsx.2021.05.019
- [Google Scholar]
- 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes - 2021. Diabetes Care. 2021;44(Suppl. 01):S15-S33.
- [CrossRef] [PubMed] [Google Scholar]
- Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(03):193-199. DOI: 10.1007/s00592-009-0109-4
- [CrossRef] [PubMed] [Google Scholar]
- Joint British Diabetes Societies (JBDS) for Inpatient Care. Management of hyperglycaemia and steroid (glucocorticoid) therapy: a guideline from the Joint British Diabetes Societies (JBDS) for Inpatient Care group. Diabet Med. 2018;35(08):1011-1017. DOI: 10.1111/dme.13675
- [CrossRef] [PubMed] [Google Scholar]
- Code mucor: guidelines for the diagnosis, staging and management of rhino-orbito-cerebral mucormycosis in the setting of COVID-19. Indian J Ophthalmol. 2021;69(06):1361-1365. DOI: 10.4103/ijo.IJO_1165_21
- [Google Scholar]
- Concensus Group. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163-170.
- [Google Scholar]
- Prevalence of co-morbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1915-1924. DOI: 10.1111/dom.14124
- [CrossRef] [PubMed] [Google Scholar]
- Is newly diagnosed diabetes a stronger risk factor than pre-existing diabetes for COVID-19 severity? J Diabetes. 2021;13(02):177-178. DOI: 10.1111/1753-0407.13125
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(05):531-538. DOI: 10.1007/s00392-020-01626-9
- [CrossRef] [PubMed] [Google Scholar]
- CORONADO investigators. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. [published correction appears in Diabetologia. 2020 Jul 2;] Diabetologia. 2020;63(08):1500-1515. DOI: 10.1007/s00125-020-05180-x
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-95. DOI: 10.1016/j.ijid.2020.03.017
- [CrossRef] [PubMed] [Google Scholar]
- Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(06):1068-1077.e3. DOI: 10.1016/j.cmet.2020.04.021
- [CrossRef] [PubMed] [Google Scholar]
- Influence of diabetes mellitus on the severity and fatality of SARS-CoV-2 (COVID-19) infection. Diabetes Obes Metab. 2020;22(10):1907-1914. DOI: 10.1111/dom.14105
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 and African Americans. JAMA. 2020;323(19):1891-1892. DOI: 10.1001/jama.2020.6548
- [CrossRef] [PubMed] [Google Scholar]
- Evidence mounts on the disproportionate effect of COVID-19 on ethnic minorities. Lancet Respir Med. 2020;8(06):547-548. DOI: 10.1016/S2213-2600(20)30228-9
- [CrossRef] [PubMed] [Google Scholar]
- Is ethnicity linked to incidence or outcomes of covid-19? BMJ. 2020;369:m1548. Published 2020 Apr 20. DOI: 10.1136/bmj.m1548
- [CrossRef] [PubMed] [Google Scholar]
- Potential metabolic and inflammatory pathways between COVID-19 and new-onset diabetes. Diabetes Metab. 2021;47(02):101204. DOI: 10.1016/j.diabet.2020.10.002
- [CrossRef] [PubMed] [Google Scholar]
- Newly diagnosed diabetes in COVID-19 patients. Prim Care Diabetes. 2021;15(01):194. DOI: 10.1016/j.pcd.2020.08.014
- [CrossRef] [PubMed] [Google Scholar]
- Proportion of newly diagnosed diabetes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Obes Metab. 2021;23(03):870-874. DOI: 10.1111/dom.14269
- [CrossRef] [PubMed] [Google Scholar]
- 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl. 01):S14-S31. DOI: 10.2337/dc20-S002
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 and diabetes: Insulin requirements parallel illness severity in critically unwell patients. Clin Endocrinol (Oxf). 2020;93(04):390-393. DOI: 10.1111/cen.14288
- [CrossRef] [PubMed] [Google Scholar]
- Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: Meta-analysis A. JAMA. 2020;324(13):1330-1341. DOI: 10.1001/jama.2020.17023
- [Google Scholar]
- RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(08):693-704. DOI: 10.1056/NEJMoa2021436
- [Google Scholar]
- SARS-CoV-2, uncontrolled diabetes and corticosteroids-an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg. 2021;20(03):418-425. DOI: 10.1007/s12663-021-01532-1
- [CrossRef] [PubMed] [Google Scholar]
- SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3(02):149-165. DOI: 10.1038/s42255-021-00347-1
- [CrossRef] [PubMed] [Google Scholar]
- Association between acute pancreatitis and COVID-19: could pancreatitis be the missing piece of the puzzle about increased mortality rates? J Invest Surg. 2022;35(01):119-125. DOI: 10.1080/08941939.2020.1833263
- [CrossRef] [PubMed] [Google Scholar]
- High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259-264. DOI: 10.1038/s41586-021-03553-9
- [CrossRef] [PubMed] [Google Scholar]
- Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthal Plast Reconstr Surg. 2021;37(02):e40-e80. DOI: 10.1097/IOP.0000000000001889
- [Google Scholar]
- A case of fatal rhino-orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID-19. Cureus. 2021;13(02):e13163. Published 2021 Feb 5. DOI: 10.7759/cureus.13163
- [CrossRef] [PubMed] [Google Scholar]
- Mucor in a viral land: a tale of two pathogens. Indian J Ophthalmol. 2021;69(02):244-252. DOI: 10.4103/ijo.IJO_3774_20
- [Google Scholar]
- The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol. 2021;14(02):305-316. DOI: 10.1038/s41385-020-00359-2
- [CrossRef] [PubMed] [Google Scholar]
- Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19. J Clin Invest. 2021;131(13):e148517. DOI: 10.1172/JCI148517
- [CrossRef] [PubMed] [Google Scholar]
- Diabetes mellitus and coronavirus disease (Covid-19) associated mucormycosis (CAM): a wake-up call from Egypt. Diabetes Metab Syndr. 2021;15(05):102195. DOI: 10.1016/j.dsx.2021.102195
- [CrossRef] [PubMed] [Google Scholar]
- T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20(09):529-536. DOI: 10.1038/s41577-020-0402-6
- [CrossRef] [PubMed] [Google Scholar]
- Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136(11):1330-1341. DOI: 10.1182/blood.2020007252
- [CrossRef] [PubMed] [Google Scholar]
- Brief review: cardiac complications and platelet activation in COVID-19 infection. Afr J Thorac Crit Care Med. 2020;26(03) Published 2020 Sep 16. DOI: 10.7196/AJTCCM.2020.v26i3.107
- [CrossRef] [PubMed] [Google Scholar]
- The potential role of dyslipidemia in COVID-19 severity: an umbrella review of systematic reviews. J Lipid Atheroscler. 2020;9(03):435-448. DOI: 10.12997/jla.2020.9.3.435
- [CrossRef] [PubMed] [Google Scholar]





