Translate this page into:
Efficacy of Phenylephrine in Preventing Hemodynamic Responses of Oxytocin during Elective Cesarean Section: A Randomized, Double-Blind, Controlled Trial
Address for correspondence Medha Mohta, MBBS, MD, MAMS, 28-B, Pocket-C, SFS Flats, Mayur Vihar Phase-III, Delhi 110096, India (e-mail: medhamohta@gmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This study compared hemodynamic changes and occurrence of complications following oxytocin administration with a prior injection of phenylephrine 100 μg or normal saline during elective cesarean section. Sixty-six healthy term parturients with uncomplicated, singleton pregnancy undergoing elective cesarean section under spinal anesthesia were studied. They received either intravenous phenylephrine 100 μg or normal saline before oxytocin 3 IU was administered over 30 seconds. Oxytocin dose was repeated depending on the adequacy of uterine tone. There was no significant change in systolic, diastolic, and mean arterial pressures during the initial 3 minutes following oxytocin administration in the phenylephrine group but a significant fall in mean and diastolic pressures in the saline group. Heart rate did not change significantly, and no significant complications occurred in either of the groups. To conclude, phenylephrine 100 μg administered before oxytocin injection maintained hemodynamic parameters better than normal saline injection during elective cesarean section.
Keywords
oxytocin
phenylephrine
cesarean section
hemodynamic changes
blood pressure
Introduction
Uterotonics are the drugs that initiate and maintain adequate uterine contractility after placental delivery, thus reducing blood loss from the site of placental attachment. Among the various agents available in clinical practice, oxytocin is used as the first line drug for prophylaxis and treatment of uterine atony.1 It is routinely administered during cesarean section and is known to decrease the incidence of postpartum hemorrhage by up to 40%.2 However, use of intravenous (IV) oxytocin may be associated with adverse hemodynamic effects such as tachycardia, hypotension, and electrocardiography (ECG) changes.3
Traditionally, large doses of IV oxytocin, that is, 5 to 10 IU, were used.4 Recent studies have proven the effectiveness of low-dose oxytocin boluses ranging from 1 to 3 IU.3,5-8 However, even this low dose can cause hypotension and tachycardia; doses between 0.5 and 3 IU have been shown to produce hypotension in 20 to 30% of patients.3
Phenylephrine, an α-adrenergic receptor agonist, is now established as the vasopressor of choice to prevent and treat postspinal hypotension during cesarean section.9 The most commonly used bolus dose is 100 μg. It results in increased blood pressure with an associated reflex decrease in heart rate (HR). Thus, phenylephrine might prove beneficial for the prevention of hypotension and tachycardia associated with oxytocin administration.
Current literature has not established the best effective phenylephrine dose for the prevention of oxytocin-induced hypotension and tachycardia. Dyer et al in 2009 demonstrated that phenylephrine 80 μg with oxytocin 2.5 IU could obtund but not abolish the adverse hemodynamic effects of oxytocin and suggested further research to find the most effective dose and timing of phenylephrine administration.6 Rumboll et al in 2015 found that prior administration of IV phenylephrine 50 μg did not prevent hypotension and tachycardia caused by a slow bolus of 3 IU oxytocin.7 As phenylephrine administration in a dose of 50 μg was found to be ineffective7 and the dose of 80 μg was partially effective in abolishing oxytocin-induced hemodynamic effects,6 it was hypothesized that administration of phenylephrine 100 μg just before oxytocin injection would be effective in preventing oxytocin-induced hypotension and tachycardia. Hence, this study was conducted with the aim of evaluating the efficacy of phenylephrine 100 μg in preventing hemodynamic responses of 3 IU oxytocin during elective cesarean section. The objectives were to study and compare the changes in HR and blood pressure and the occurrence of complications following oxytocin administration with a prior injection of phenylephrine 100 μg or normal saline during elective cesarean section.
Materials and Methods
This was a prospective, randomized, double-blind, placebo-controlled trial conducted after obtaining approval from the institutional ethics committee. The recruitment was conducted from November 2015 to February 2017, and a written informed consent was obtained from all the patients. The trial was registered prospectively at www.ctri.nic.in.
A total of 66 healthy term parturients carrying an uncomplicated, singleton pregnancy who were planned for elective cesarean section under spinal anesthesia were included. The study excluded the following patients: those in active labor; those with ruptured amniotic membranes; those with maternal complications such as preeclampsia, diabetes mellitus, cardiovascular disease, and cerebrovascular disease; those with known risk factors for postpartum hemorrhage such as multiple gestation, abnormal placentation, uterine fibroid, macrosomia, hydramnios, history of postpartum hemorrhage, and uterine atony; and those with contraindications for spinal anesthesia such as infection in lumbar area, coagulation abnormalities, autonomic neuropathy, spinal deformities, other neurological diseases, and hypovolemia due to any cause.
The patients were randomly divided into two groups of 33 each using computer-generated random number table, group P and group NS, as per the administered drug. Sealed envelopes were prepared according to the random number allocation to maintain allocation concealment. In group P, phenylephrine 100 μg in 1-mL volume was injected before oxytocin administration; whereas, in group NS, 1-mL saline was injected before oxytocin.
The patients fasted for at least 8 hours and received aspiration prophylaxis in the form of ranitidine and metoclopramide before being shifted to the operating room. In the operating room, baseline maternal HR and noninvasive blood pressure (NIBP) were recorded with a wedge placed under the right buttock. IV coloading with 15 mL/kg of Ringer's lactate solution was started. Subarachnoid block was performed with a midline approach at L2–L3 or L3–L4 vertebral interspace using a 25-gauge spinal needle, and 2.2 or 2.0 mL of hyperbaric 0.5% bupivacaine was injected if the patient's height was ≥150 cm or <150 cm, respectively.
Intraoperative monitoring included continuous ECG, HR, NIBP, and pulse oximetry. HR and NIBP were noted before giving spinal anesthesia and then every minute after spinal injection until 10 minutes after delivery of baby. Hypotension was defined as a fall of ≥20% from the baseline mean arterial pressure (MAP) and was treated with phenylephrine 100 μg. Hypertension was defined as a rise of more than 20% from the baseline MAP. IV glycopyrrolate 0.2 mg was administered at HR < 60 bpm with hypotension or HR < 45 bpm irrespective of MAP value.
At the time of umbilical cord clamping, the test drug was administered. Following this, all the patients received a slow injection of oxytocin 3 IU diluted to 5 mL over 30 seconds. The readings of HR and NIBP just before the injection of test drug were considered as the baseline values for subsequent changes. Thereafter, HR, systolic blood pressure (SBP), diastolic blood pressure (DBP), and MAP were monitored every minute for the next 10 minutes. Patients who received phenylephrine for the treatment of hypotension within 2 minutes before delivery or whose MAP at the time of delivery was more than 20% from initial baseline value were excluded. In this situation, the same randomization code was used for the next patient.
Uterine tone was graded 3 minutes after delivery as adequate or inadequate by the obstetrician. If inadequate, a rescue dose of oxytocin 3 IU was given slowly. Two such rescue doses were given at 3-minute interval, if required. An infusion of oxytocin at a rate of 0.08 IU/minute was started after 10 minutes of initial oxytocin injection. If uterine tone was unsatisfactory despite three oxytocin boluses, carboprost injection was administered in consultation with the obstetrician.
Intraoperative blood loss was estimated by the volume of blood in the suction bottle and the number of soaked sponges. The timings of skin incision, uterine incision, and delivery of baby; uterine tone at 3 minutes; intraoperative requirement of uterotonics; intraoperative blood loss; and any complications observed, such as nausea, vomiting, and ECG changes were recorded.
The primary outcome measure was peak changes in MAP and HR during the initial 3 minutes following oxytocin administration, whereas the secondary outcomes included changes in SBP and DBP and any complications observed after delivery.
Sample Size Calculation
Sample size was calculated based on the primary outcomes, that is, peak changes in HR and MAP following oxytocin administration. Considering standard deviation (SD) of peak change in MAP after oxytocin administration to be around 10%6 and taking 10% change as clinically significant, the sample size required at 90% power and 2.5% level of significance was 27 in each group. Similarly, for peak HR changes, considering SD of change to be around 20%6 and taking 20% change in peak HR as clinically significant, the sample size again turned out to be 27 per group. To compensate for possible exclusions after randomization, 20% of this number was added and therefore 33 patients were studied in each group. Because of two primary outcomes, that is, HR and MAP, the type I error was reduced to 2.5%.
Statistical Analysis
The statistical analysis was performed using SPSS statistical software (version 20.0). The data were presented as mean (SD) or as median (interquartile range). Unpaired t-test was used to compare demographic profile, other patient characteristics, baseline hemodynamic parameters, various time intervals, phenylephrine dose and time before delivery, and hemodynamic variables at different time points before and after delivery. Dunnett's test and Tukey's test were applied to analyze the peak changes in various hemodynamic parameters. Value of p < 0.05 was considered significant.
Results
A total of 78 patients met the inclusion criteria, of which 66 patients were included for the trial. The CONSORT (Consolidated Standards of Reporting Trials) flow diagram is shown in ►Fig. 1.
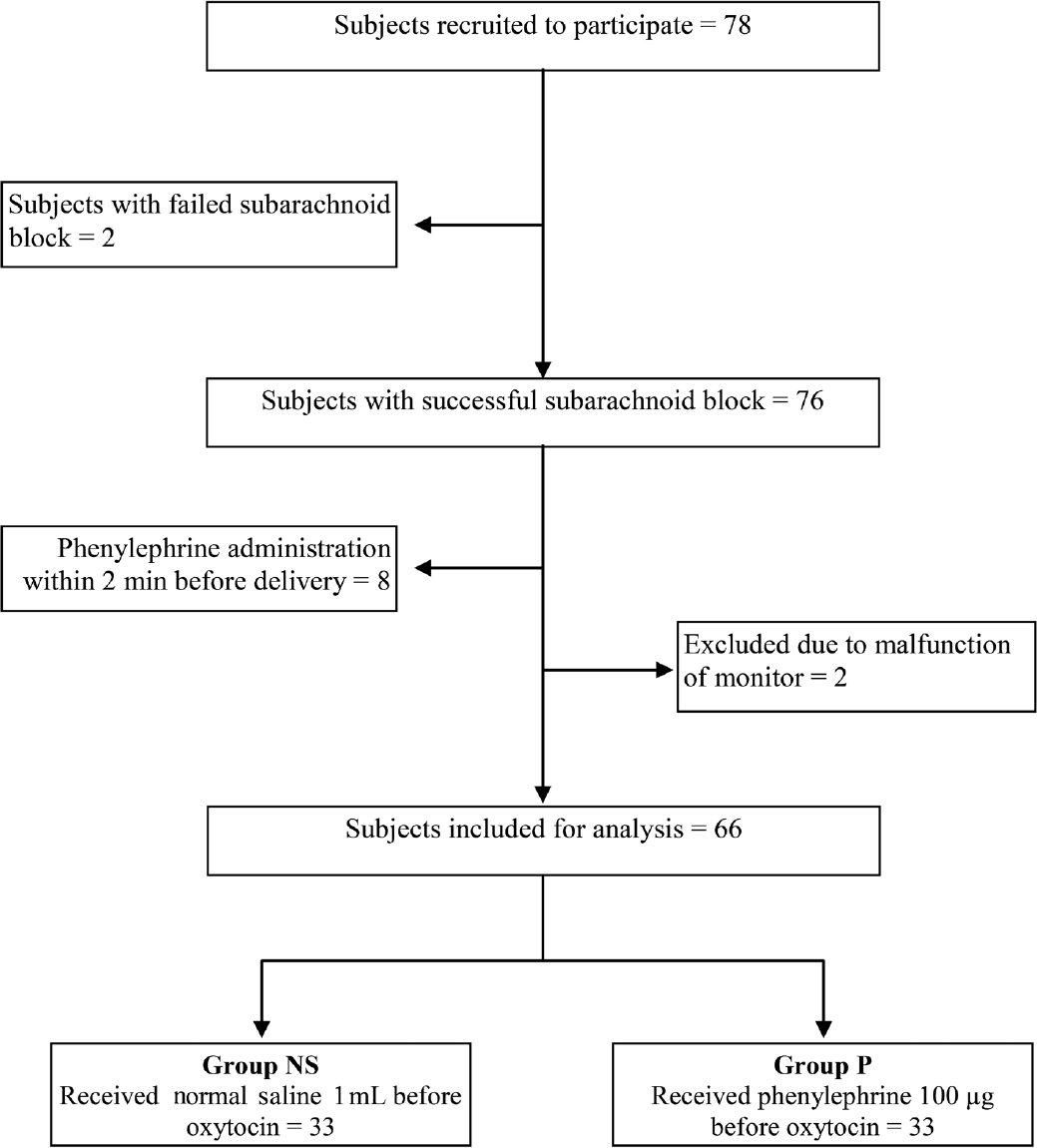
- Consolidated Standards of Reporting Trials flow diagram.
Demographic profile, various intraoperative time intervals, and other patient variables such as gestational age, fluid till delivery, phenylephrine dosage and time of the last dose before delivery, spinal to cord clamp interval, and baseline hemodynamic parameters were comparable in the two groups (►Table 1).
| Group NS (n = 33) | Group P (n = 33) | p-Value | |
|---|---|---|---|
| Age (years) | 26.1 ± 3.8 | 25.4 ± 2.9 | 0.421 |
| Weight (kg) | 60.6 ± 5.6 | 62.7 ± 7.4 | 0.180 |
| Height (cm) | 151.0 ± 3.9 | 152.1 ± 3.2 | 0.220 |
| Period of gestation (weeks) | 38.2 ± 1.1 | 38.0 ± 1.1 | 0.435 |
| Fluid till delivery (mL) | 722.1 ± 63.0 | 737.9 ± 77.1 | 0.366 |
| Total phenylephrine before delivery (μg) | 160.0 ± 82.8 | 170.6 ± 99.0 | 0.746 |
| Time of last phenylephrine dose before delivery (minutes) | 6.9 ± 2.8 | 5.5 ± 1.9 | 0.097 |
| Total oxytocin (IU) | 3.5 ± 1.2 | 3.9 ± 1.6 | 0.294 |
| Blood loss (mL) | 506.1 ± 90 | 563.6 ± 95.4 | 0.014a |
| Spinal to cord clamp interval (minutes) | 11.1 ± 4.4 | 10.4 ± 3.5 | 0.480 |
| Baseline HR (beats/min) | 88.1 ± 13.7 | 92.4 ± 14.8 | 0.225 |
| Baseline MAP (mmHg) | 94.1 ± 7.0 | 91.2 ± 7.1 | 0.096 |
| Baseline SBP (mmHg) | 123.8 + 9.8 | 121.3 ± 10.0 | 0.300 |
| Baseline DBP (mmHg) | 76.2 ± 8.5 | 71.7 ± 9.8 | 0.050 |
| Hypotension value (mmHg) | 75.3 + 5.6 | 73.0 ± 5.6 | 0.096 |
| Hypertension value (mmHg) | 112.6 ± 8.6 | 108.9 ± 8.4 | 0.083 |
Abbreviations: DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; SBP, systolic blood pressure. Note: Values are presented as mean ± standard deviation.
aStatistically significant.
Similar treatment was provided for the management of hypotension, with 15 patients in group NS and 17 in group P developing hypotension and receiving phenylephrine boluses before delivery. Thus, both groups were comparable till the time of intervention, that is, test drug administration at cord clamping.
The uterine tone assessed after 3 minutes of initial oxytocin administration was adequate in all but three patients in each group. The total dose of oxytocin administered was comparable in both the groups (►Table 1). None of the patients required carboprost administration.
The estimated blood loss was significantly higher in patients receiving phenylephrine than those receiving normal saline (►Table 1).
Hemodynamic Changes
The HR, MAP, SBP, and DBP values did not differ significantly between the two groups at any time point from just before drug administration till up to 10 minutes after delivery of the baby. The trends of HR and MAP are shown in ►Figs. 2 and 3.
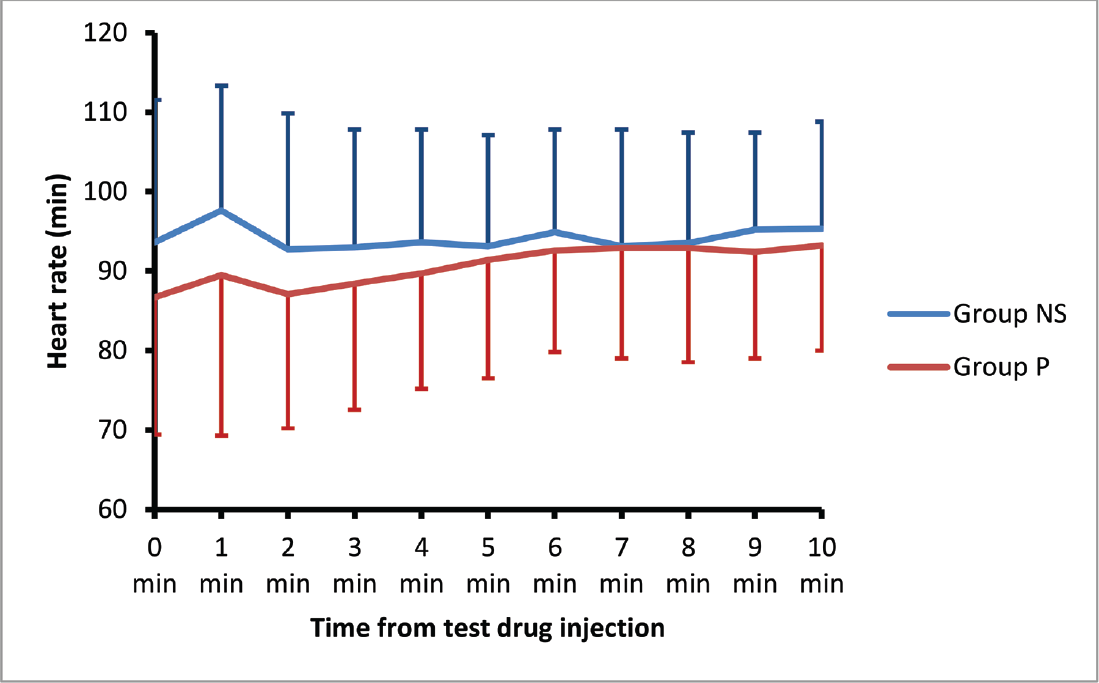
- Heart rate trends after test drug and oxytocin administration.
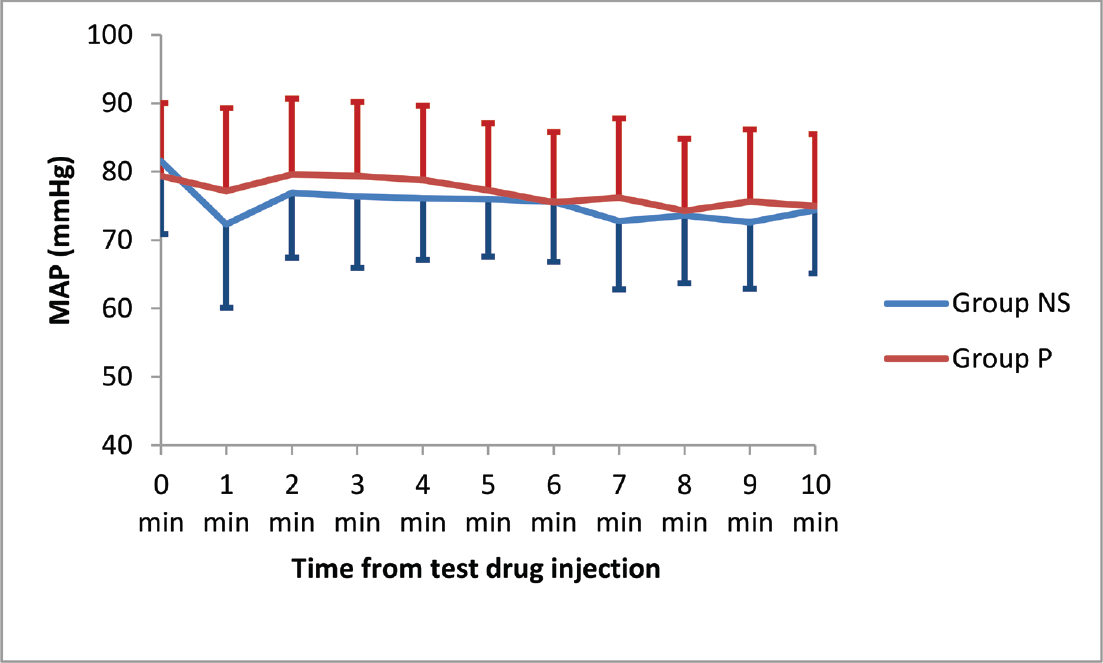
- Mean arterial pressure trends after test drug and oxytocin administration.
►Table 2 shows peak changes in hemodynamic variables within 3 minutes after oxytocin administration. The peak changes in HR within 3 minutes of oxytocin administration remained statistically similar between as well as within both groups (p = 0.333).
| Group NS | Group P | Intergroup p-value | |||||
|---|---|---|---|---|---|---|---|
| Mean at oxytocin injection | Peak value | Peak change | Mean at oxytocin injection | Peak value | Peak change | ||
| Heart rate (beats/minutes) | 93.6 ± 17.9 | 95.8 ± 18.6 | +2.18 | 86.73 ± 17.3 | 88.5 ± 20.1 | +1.79 | 0.078 |
| Mean BP (mm Hg) | 81.5 ± 10.6 | 70.6 ± 11.0 | –10.9 | 79.4 ± 10.6 | 78.0 ± 14.3 | –1.4 | 0.053 |
| Systolic BP (mm Hg) | 113.2 ± 14.0 | 103.9 ± 15.1 | –9.3 | 111.1 ± 13.0 | 113.0 ± 16.4 | +1.9 | 0.043 |
| Diastolic BP (mm Hg) | 60.3 ± 11.4 | 51.1 ± 12.7 | –9.2 | 57.8 ± 10.1 | 56.0 ± 15.5 | –1.8 | 0.386 |
Abbreviation: BP, blood pressure.
Note: Values are presented as mean ± standard deviation.
The peak changes in MAP as well as DBP showed a statistically significant fall in group NS within the initial 3 minutes after oxytocin administration (p = 0.000). These changes were not significant within group P. On intergroup comparison, the difference could not achieve statistical significance (►Table 2).
Although the fall in SBP values in group NS appeared to be large, this change was statistically insignificant within the group (p = 0.054). The peak change within group P was also not significant. However, on intergroup analysis, the peak change in SBP was found to be significantly higher in group NS than in group P (►Table 2).
Complications
Two patients in group NS and none in group P developed nausea during the study period. No ECG changes or other complications were noted in any patient in either group during the study period.
Discussion
The results of this study demonstrated that phenylephrine 100 μg injected just before the administration of oxytocin 3 IU prevented a significant fall in MAP and DBP during the subsequent 3-minute period when compared with baseline values; however, the intergroup difference in peak change within 3 minutes following oxytocin administration in patients receiving phenylephrine or saline pretreatment was statistically significant only for SBP. No significant effect on HR could be seen with or without phenylephrine administration.
Earlier, oxytocin was used in high doses of 5 to 10 IU bolus. However, use of such high bolus doses for prophylaxis of uterine atony has been questioned.5,10 Higher doses of oxytocin are probably not necessary because the concentration of myometrial receptors reaches its peak at term under the influence of estrogen. Therefore, lower doses may be equally effective and appear to be associated with fewer and less severe maternal side effects. Tsen and Balki proposed a “rule of threes” for the administration of uterotonics.10 The same protocol was followed in this study. The total oxytocin requirement was found to be low and comparable in both groups. It was 3.5 ± 1.2 IU in group NS and 3.9 ± 1.6 IU in group P (p = 0.294).
Oxytocin is known to cause tachycardia when administered as IV bolus in conventional doses of 5 to 10 IU.3 The incidence reduces with lower doses but even these lower doses have been seen to cause adverse hemodynamic effects.3 However, in this study, the administration of oxytocin did not cause any clinically significant tachycardia in either of the groups. This could be because we used a low dose of oxytocin 3 IU administered as a slow IV bolus over 30 seconds.
Phenylephrine being a direct α-adrenergic agonist increases the peripheral vascular resistance, thus preventing oxytocin-induced hypotension.6 This is evident in our results as the peak changes in blood pressure values were clinically significant in only group NS.
Our results are supported by the work of Dyer et al who studied hemodynamic effects of coadministration of phenylephrine 80 μg with oxytocin 2.5 IU during spinal anesthesia for elective cesarean delivery.6 The mean peak percentage change in MAP was 2.99% in group P and–28.9% in group NS. They concluded that phenylephrine obtunded the hemodynamic effects of oxytocin.
Rumboll et al used phenylephrine 50 μg or saline before injecting oxytocin 3 IU and observed a mean peak percentage change in SBP to be–16.9% in the phenylephrine group and–19.0% in the saline group (p = 0.44).7 They concluded that phenylephrine 50 μg was not effective in preventing oxytocin-induced hypotension. The low dose of phenylephrine used by Rumboll et al was probably the reason behind the lack of efficacy in their study.
Recently, Gangadharaiah et al studied the effects of coadministration of phenylephrine 50 μg, phenylephrine 75 μg, or saline with oxytocin on the prevention of hypotension during cesarean section.11 They demonstrated a significant fall in MAP after oxytocin infusion in all the three groups; however, the magnitude of fall was minimum with phenylephrine 75 μg. HR remained comparable with no incidence of bradycardia in any of the groups. Their study design was different from ours in many aspects. They included patients undergoing both elective and emergency cesarean sections. The oxytocin requirements and hemodynamic responses may be quite different in emergency cesareans. They assessed uterine tone only at the end of uterine closure, whereas we assessed it every 3 minutes and administered additional uterotonics accordingly. Their method of oxytocin administration was also different from ours.
In this study, patients in active labor and those with ruptured membranes were excluded as these patients may have been exposed to oxytocin before coming for lower segment cesarean section, resulting in oxytocin receptor desensitization.12 This may lead to higher oxytocin requirements and greater use of second-line uterotonics. Phenylephrine was also used before delivery to treat any hypotensive episodes. As this could influence the hemodynamic parameters following oxytocin administration, the patients receiving phenylephrine within 2 minutes before delivery were excluded. Eight such patients were excluded because of this reason. Repeat phenylephrine injection at cord clamping could have led to exaggerated hypertension; hence, it was decided to exclude and not administer the test drug at the time of cord clamping to the patients whose MAP at the time of delivery was more than 20% from the initial baseline value. However, no patient in either group had MAP more than 20% from the initial baseline value at the time of delivery. Two patients in group NS had to be excluded, as the vital sign monitor developed an error during the conduct of the study, resulting in some missed values of HR and blood pressure. The estimated blood loss was found to be more in group P. Although this difference was statistically significant (p = 0.014), it did not appear to be clinically significant.
This study has certain limitations. First, the intraoperative blood loss was measured by the volume of blood collected in the suction bottle and the number of soaked sponges. This was not a very accurate method of blood loss calculation due to mixing of liquor in the suction bottle at the time of delivery. However, it served good as a rough estimate. Second, the study included only elective cesarean sections. Therefore, the results cannot be generalized and may not be extrapolated to patients undergoing emergency cesarean sections.
To conclude, phenylephrine 100 μg administered before oxytocin injection 3 IU over 30 seconds maintains hemodynamic parameters better than normal saline injection during elective cesarean section.
Conflict of Interest
None declared.
References
- WHO recommendations for the prevention and treatment of postpartum haemorrhage. 2012 Available at: apps.who.int/iris/bitstream/10665/75411/1/9789241548502_eng.pdf (accessed )
- [Google Scholar]
- Effects of synthetic oxytocin with and without preservatives upon coronary blood flow in the dog. J Pharmacol Exp Ther. 1969;165(02):258-266.
- [Google Scholar]
- Minimum effective bolus dose of oxytocin during elective caesarean delivery. Br J Anaesth. 2010;104(03):338-343.
- [CrossRef] [PubMed] [Google Scholar]
- An Australian and New Zealand survey of practice of the use of oxytocin at elective caesarean section. Aust N Z J Obstet Gynaecol. 2010;50(01):30-35.
- [CrossRef] [PubMed] [Google Scholar]
- Oxytocin for labour and caesarean delivery: implications for the anaesthesiologist. Curr Opin Anaesthesiol. 2011;24(03):255-261.
- [CrossRef] [PubMed] [Google Scholar]
- Hemodynamic effects of ephedrine, phenylephrine, and the coadministration of phenylephrine with oxytocin during spinal anesthesia for elective cesarean delivery. Anesthesiology. 2009;111(04):753-765.
- [CrossRef] [PubMed] [Google Scholar]
- The use of phenylephrine to obtund oxytocin-induced hypotension and tachycardia during caesarean section. Int J Obstet Anesth. 2015;24(04):297-302.
- [CrossRef] [PubMed] [Google Scholar]
- A Randomized, double-blinded trial of a “rule of threes” algorithm versus continuous infusion of oxytocin during elective cesarean delivery. Anesthesiology. 2015;123(01):92-100.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention of maternal hypotension after regional anaesthesia for caesarean section. Curr Opin Anaesthesiol. 2010;23(03):304-309.
- [CrossRef] [PubMed] [Google Scholar]
- Oxytocin protocols during cesarean delivery: time to acknowledge the risk/benefit ratio? Int J Obstet Anesth. 2010;19(03):243-245.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of co-administration of different doses of phenylephrine with oxytocin on the prevention of oxytocin-induced hypotension in caesarean section under spinal anaesthesia: a randomised comparative study. Indian J Anaesth. 2017;61(11):916-922.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular regulation of the oxytocin receptor in peripheral organs. J Mol Endocrinol. 2003;30(02):109-115.
- [CrossRef] [PubMed] [Google Scholar]





