Translate this page into:
Granulomas co-occurring in malignancies – tale of etiological relationship
* Corresponding author: Dr. Sacheeta Babuta, Department of Pathology, Rohilkhand Medical College and Hospital, Pilibhit Bypass Road, Bareilly, India. Email: babuta.sacheeta@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Babuta S, Garg C, Vinayakamurthy S, Agrawal R, Agarwal A. Granulomas co-occurring in malignancies – tale of etiological relationship. Ann Natl Acad Med Sci (India). 2024;60:139–46. doi: 10.25259/ANAMS-2022-12-9-(804)
Abstract
Objectives
To determine the possible aetiopathogenesis of co-occurrence of granulomas with different malignancies in different body sites.
Material and methods
All cases with granuloma formation observed in draining lymph nodes or in the primary site of malignant tumors were included in the present study. After routine histopathology examination, modified Ziehl-Neelsen (ZN) staining for Mycobacteria was carried out in all the cases. Detailed history, especially of Tuberculosis, sarcoidosis, neoadjuvant chemotherapy, radiation, or previous procedure, was recorded.
Results
11 out of 35 cases (31.4%) had granulomas within the primary tumor, while 24 cases out of 35 (68.6%) showed nodal granulomas. Of the 24 cases, 5 cases had nodal metastatic tumor deposits. Also, necrotizing granulomas with AFB were significantly more as compared to AFB in non-necrotizing granulomas (p value of 0.05). Of the total cases, 20% had an attributed risk factor. Three cases received neoadjuvant chemotherapy, and three had a history of systemic tuberculosis, while one case had associated Crohn’s disease.
Conclusion
We recommend to characterize granulomas as necrotizing/nonnecrotizing, confluent/discrete, tumor deposit present/absent; prior history of systemic/local illnesses (like SS, TB, fungal infections, IBD, etc.); prior history of CT/RT; and to follow routine ZN staining in all cases of granulomas with malignancy.
Keywords
Granulomas
Malignancy
Sarcoidosis
Tuberculosis
ZN Staining
INTRODUCTION
Granulomatous inflammation is one of the most common diagnoses in pathology in India.1 In patients with malignancy, granulomatous lesions may quite often be a fortuitous finding, especially in primary diagnostic biopsies.2 These granulomas are included as a part of the primary cancer or are present in the lymph nodes that drain the tumor.3 The exact etiopathogenesis or the therapeutic implications in patients with malignancy remain obscure.1,3–5
In a developing country like India, where the incidence of tuberculosis (TB) is high, it is sometimes difficult to distinguish between a concomitant TB and a nonspecific granulomatous response with focal necrosis.4 Tuberculous etiology should mandatorily be ruled out before any other cause is considered.3,6
Granulomatous inflammation is a chronic inflammatory reaction characterized by microscopic aggregation of activated macrophages with an epithelioid appearance. These may or may not be associated with mononuclear cells, classical multinucleate Langhans’ giant cells, or necrosis. The presence of granulomatous inflammation could be due to infective or noninfective causes, while granulomas can be necrotizing or nonnecrotizing.3 A tubercular granuloma is discrete with the presence of Langhans’ multinucleated giant cells, lymphocytes, epithelioid cells, and caseous necrosis, while the typical sarcoid granuloma is coalescent and noncaseating with the presence of plump epithelioid cells and giant cells (both foreign body or Langhans’ type).1,4
Granulomas in the lymph nodes draining the primary tumor (with or without metastatic cancer) have been variously labeled as sarcoid-like or a sarcoid reaction.1 Gorton and Linell were the first to report cases with sarcoid-like granulomatous inflammation in lymph nodes draining carcinomas.7,8 Formation of a granuloma has also been observed in many malignancies treated with neoadjuvant chemotherapy or radiation therapy, especially in cancers of the breast, stomach, colonic, or larynx.3,6
Infections due to bacteria, mycobacteria, spirochaetes, viruses or fungi, local irritants, Crohn’s disease, Whipple’s disease, and cirrhosis have been found to be the different causes of granuloma formation in patients with malignancies. Amongst these, mycobacterial infection is probably one of the most frequently presenting infections asymptomatically.2
Several studies have demonstrated a relationship between systemic sarcoidosis (SS) and malignant tumors. Sarcoidosis can occur any time before, during, or after the cancer is diagnosed. Studies have shown that patients previously diagnosed with SS have a 2–3 times higher risk of developing malignancy in the near future, and the reason behind this is not well defined.9
The present study was thus undertaken to describe the possible etiopathogenesis of co-occurrence of granulomas with different malignancies at different body sites.
MATERIAL AND METHODS
The present study was conducted at the Department of Pathology of a tertiary care center with a dedicated oncology unit. A retrospective observational study was planned. All cases with granuloma formation seen either in the draining lymph nodes or in the primary site of malignant tumors were included in the study. All benign tumors or inflammatory conditions with or without granulomatous inflammation were excluded from the present study. Institutional Ethics Committee (IEC) approval (Reference No. IEC/RMCH/86/2022/Aug) was obtained for this study with the waiver of consent.
Thirty-five cases met the inclusion criteria during the last four years. The required patients’ data were retrieved from the medical records section and analyzed. In addition to histopathology reporting, the cases were re-analyzed for granuloma characteristics, and Ziehl–Neelsen (ZN) stain was performed on all the paraffin blocks. The granulomas observed were characterized under the following subtypes:
Necrotizing or nonnecrotizing granulomas
Lymph node granuloma with or without tumor deposits
Granuloma in the primary tumor and draining lymph nodes.
RESULTS
The site-wise distribution of cases of granuloma with malignancies is depicted in Figure 1-5.
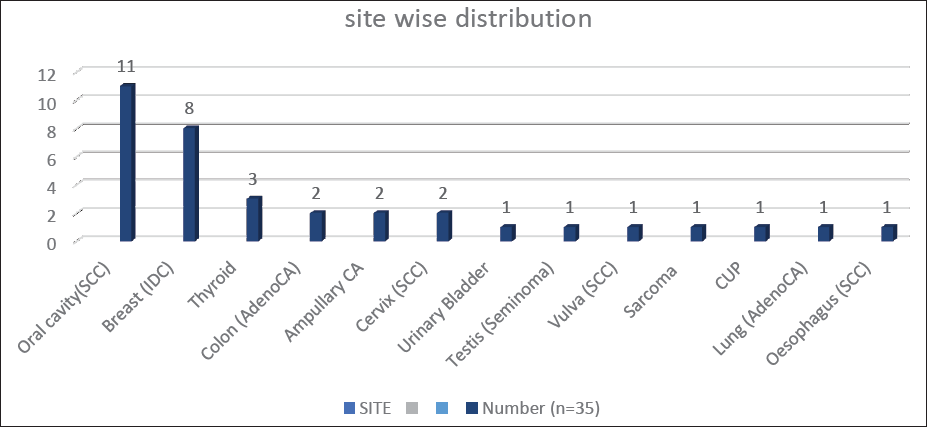
- Site-wise distribution of cases of granuloma co-occurring with malignancies (SCC: Squamous Cell Carcinoma; IDC: Invasive Ductal Carcinoma; CUP: Carcinoma Unknown Primary); Peri/Ampullary CA(Adeno CA); Urinary bladder(High grade papillary CA); Sarcoma (Spindle cell).
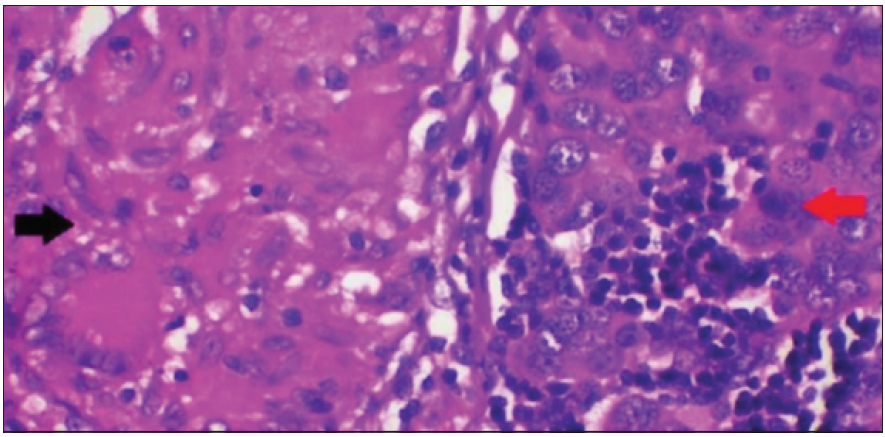
- Epithelioid granuloma (black arrow) with invasive breast carcinoma (red arrow) (Haematoxylin & Eosin ×400).
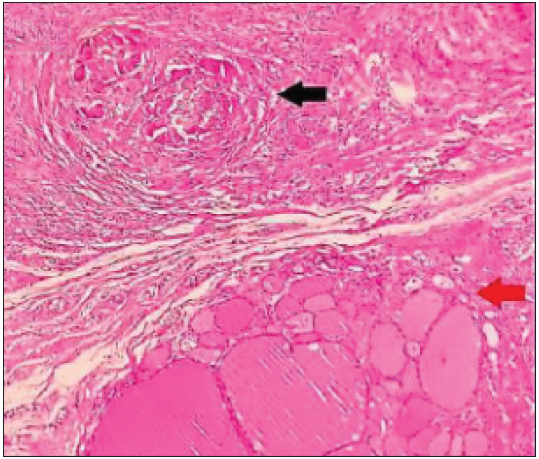
- Epithelioid granuloma (black arrow) with encapsulated follicular patterned neoplasm thyroid (red arrow) (Haematoxylin & Eosin ×100).
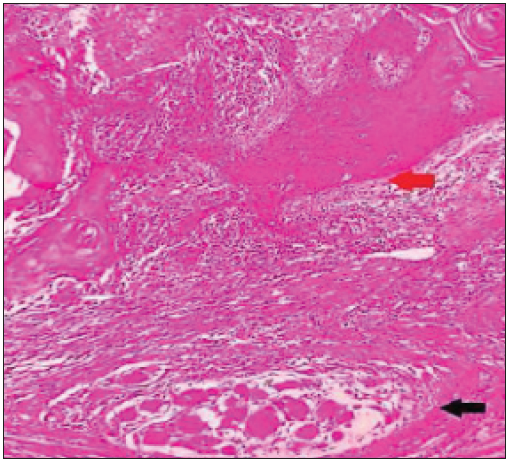
- Epithelioid granuloma (black arrow) with oral squamous cell carcinoma (red arrow) (Haematoxylin & Eosin ×100).
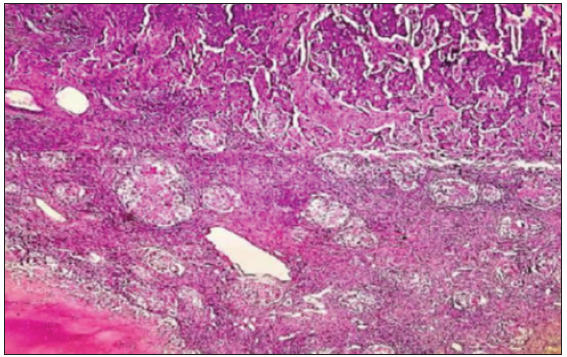
- Necrotizing granuloma in lymph nodes with metastatic deposits from invasive breast carcinoma (Haematoxylin & Eosin ×40).
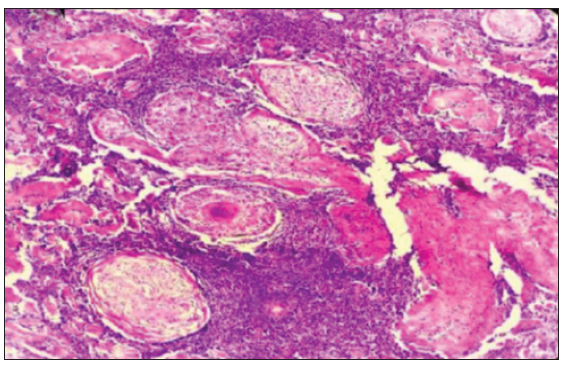
- Necrotizing granuloma in lymph node without metastatic deposits in a case of oral squamous cell carcinoma (Haematoxylin & Eosin ×100).
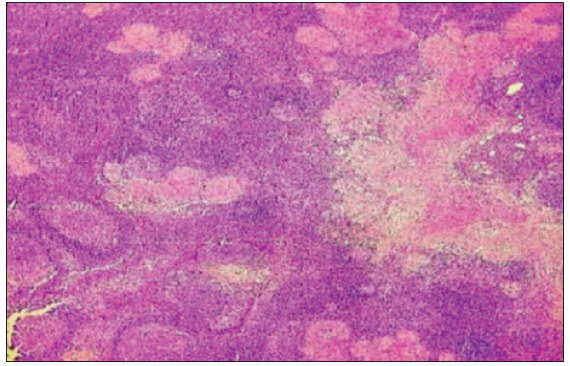
- Necrotizing discrete tuberculoid-like granuloma in lymph nodes (Haematoxylin & Eosin ×40).
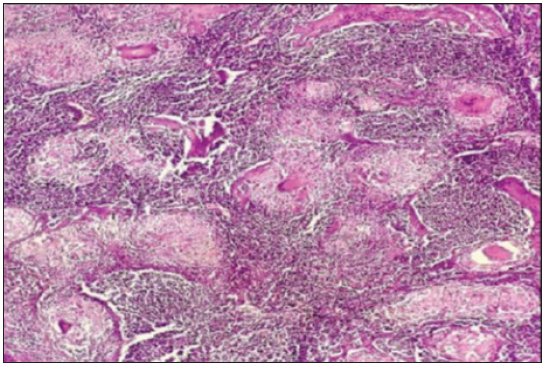
- Nonnecrotizing coalescent sarcoid-like granuloma in lymph nodes (Haematoxylin & Eosin ×100).
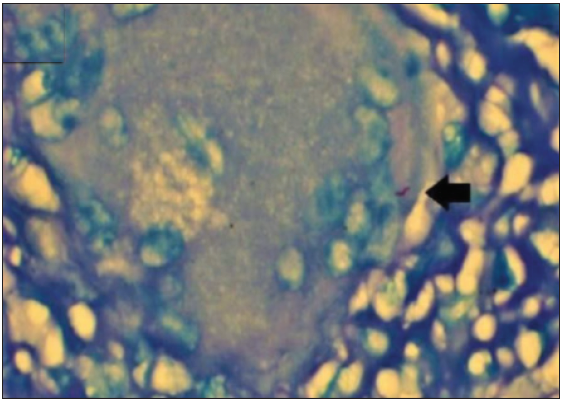
- AFB positivity in a necrotizing granuloma in a case of invasive breast cancer (ZN stain ×1000; oil immersion). Pink, rod shaped acid fast bacilli , depicting AFB positivity (Black arrow). (ZN: Ziehl-Neelsen; AFB: Acid Fast Bacilli)
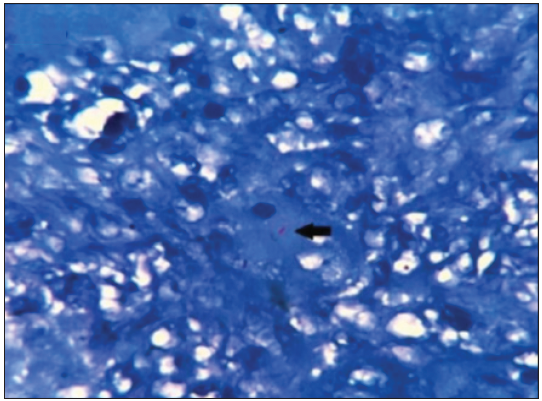
- AFB positivity in a necrotizing granuloma in a case of carcinoma cervix (ZN stain ×400). Pink, rod shaped acid fast bacilli , depicting AFB positivity (Black arrow). (ZN: Ziehl-Neelsen; AFB: Acid Fast Bacilli)
Eleven out of the 35 cases (31.4%) had granulomas within the primary tumor while 24 out of 35 cases (68.6%) showed nodal granulomas in the tumor-draining lymph nodes [Table 1].
| Sites | Cases with granuloma in lymph nodes | Cases with granuloma in the primary tumor | Total |
|---|---|---|---|
| Oral cavity | 9 | 2 | 11 |
| Breast | 7 | 1 | 8 |
| Thyroid | 0 | 3 | 3 |
| Colon | 1 | 1 | 2 |
| Periampullary/ampullary | 2 | 0 | 2 |
| Cervix | 1 | 1 | 2 |
| Urinary bladder | 0 | 1 | 1 |
| Testis | 0 | 1 | 1 |
| Vulva | 0 | 1 | 1 |
| Hand | 1 | 0 | 1 |
| Carcinoma unknown primary (lymph nodes) | 1 | 0 | 1 |
| Lung | 1 | 0 | 1 |
| Esophagus | 1 | 0 | 1 |
| Total | 24 (68.6%) | 11 (31.4%) | 35 (100%) |
| Parameters | Distribution/frequency | Percentage | Total | |
|---|---|---|---|---|
| 1) Histological type | Adenocarcinoma | 16 | 51.6 | 100% (n = 31)* |
| Squamous cell carcinoma | 15 | 48.4 | ||
| 2) ZN staining | AFB positive | 2 | 5.7 | 100% (n = 35) |
| AFB negative | 33 | 94.3 | ||
| 3) Types of granuloma | Necrotizing tuberculoid-like granulomas | 13 | 37.1 | 100% (n = 35) |
| Nonnecrotizing sarcoid-like granulomas | 22 | 62.9 | ||
| 4) Sites of granuloma | Presence of granuloma in primary tumor | 11 | 31.4 | 100% (n = 35) |
| Presence of granuloma in lymph node | 24 | 68.6 | ||
| 5) Presence of granuloma in lymph node | Lymph node with tumor deposits | 5 | 20.8 | 100% (n = 24)** |
| Lymph node without tumor deposits | 19 | 79.2 | ||
| 6) Post-neoadjuvant or chemotherapy related | Cases with prior neoadjuvant or chemotherapy | 3 | 8.6 | 100% (n = 35) |
| Cases without prior chemo or neoadjuvant therapy | 32 | 91.4 | ||
| 7) Past history | Tuberculosis | 3 | 8.6 | 100% (n = 35) |
| Inflammatory bowel disease | 1 | 2.9 | ||
| Others (Any systemic sarcoidosis or prior FNAC/Biopsy procedure done) | 0 | 88.5 | ||
A comparison of different parameters with respect to the co-occurrence of granulomas in cases of malignancies is shown in Table 2. Correlation of adenocarcinoma and squamous cell carcinoma was done in patients presenting with concurrent granulomas. Cases of spindle cell sarcoma (hand), seminoma testis, metastatic carcinoma, carcinoma of unknown primary in lymph node, and high-grade papillary urothelial carcinoma urinary bladder were not included in the comparative analysis. Table 2 also shows ZN staining results, distribution of necrotizing and nonnecrotizing granulomas, etc. History of neoadjuvant chemotherapy, past history of systemic TB, and history of IBD in a case of colon adenocarcinoma were taken, none of the cases had any history of SS or prior Fine needle aspiration cytology (FNAC)/Biopsy procedure done.
Furthermore, in the present study, we observed that acid fast bacilli (AFB) positivity was higher in necrotizing granulomas as compared to AFB in nonnecrotizing granulomas (p-value = 0.05). Of the total 35 cases, 2 cases showing positivity for AFB by ZN staining also had granulomas in the adjoining draining lymph nodes. The comparison of nodal versus extranodal AFB positivity was statistically insignificant (p-value = 0.84).
In our study, of the total 35 cases, 11 (31.43%) cases had the presence of granulomas in the primary tumor, of which 3 (8.57%) had necrotizing tuberculoid-like granulomas while 8 had nonnecrotizing sarcoid-like granulomas. Among the 24 cases with nodal granulomas, 14 (58.33%) cases had, nonnecrotizing sarcoid-like granulomas, and 10 (41.67%) cases had necrotizing tuberculoid-like granulomas. The p-value (0.65) for this distribution was statistically insignificant.
DISCUSSION
The presence of granuloma and malignancy at the same time is a rare occurrence, and it carries with it a diagnostic as well as a therapeutic dilemma. In a TB endemic and resource-limited nation like ours, a big question is whether to further investigate these cases or take these granulomas (especially the necrotizing ones) as sufficient to diagnose TB unless proven otherwise. There have been several case reports and studies describing granulomas at different cancer sites in the body. Most studies have described single cancer sites. In our study, the common three sites were oral squamous cell carcinoma (31.4%), breast carcinoma (22.8%), and papillary thyroid carcinoma (8.5%). Overall, a total of 51.6% cases of adenocarcinoma (breast, thyroid, colon, GIT, lung, etc.) and 48.4% cases of squamous cell carcinomas (oral and cervix) were included.
In one study, squamous cell carcinoma was considered to be more commonly associated with granulomas, possibly owing to more number of head and neck surgeries in the given institute.3 To date, we have not been able to find any other study in English literature that has discussed such a comparison.
Another study showed one case of papillary carcinoma thyroid having coexistence of metastatic deposits and TB in the cervical lymph nodes.10 While in our study, we reported two such cases with no history of TB along with AFB negativity.
The etiopathogenesis of these granuloma formations is multifactorial, and the following causes have been found in different studies like: neoadjuvant therapy-related, foreign body reaction owing to the necrotic tumor, or previous procedure like FNAC/biopsy; an associated systemic or local illness like TB, sarcoidosis, inflammatory bowel disease, or fungal infection; idiopathic causes, etc.1,3,4
In the majority of the cases, no definite cause has been found by researchers, and the etiology remains obscure. In these cases, granuloma formation is due to T-cell-mediated (type IV hypersensitivity) immunological reaction to soluble antigens shed by the tumor or tumor-derived debris,11 which leads to a granulomatous response, whereas others attribute it to the persistence of a non-degradable product like keratin/psammoma bodies/mucin, etc.12
Diligent history taking and routine use of ZN stain were done in all cases. Owing to this, we observed 20% of patients with one or the other etiopathogenic factor to which a coexisting granuloma formation could be attributed. Three out of 35 cases (8.6%) had a prior history of neoadjuvant chemotherapy, which may have induced granuloma formation in the residual tumor. Studies by other researchers have substantiated the occurrence of granulomatous inflammation in post-neoadjuvant chemo/radiotherapy/radioisotope therapies for various carcinomas soon after or later in the course of the disease.2-4,6 Three out of 35 (8.6%) cases in the present study had a history of TB, and two of these showed the presence of AFB within the associated necrotizing granulomas. Previous authors from India performed ZN staining in few cases of granulomas with malignancy, but they could not completely rule out tuberculosis as a cause of the primary malignancy associated with granulomas in spite of negative ZN stain result.3,6 In a study from India, three out of seven cases showed ZN positivity for AFB.13 The specificity of ZN microscopy is high (78.5%) but sensitivity is imperfect (22.2%).14
Thus, although negative cases do not rule out TB, there is a possibility of exacerbation of TB due to immune suppression and cancer therapy in such patients. Hence, a prior history, inexpensive ZN stain, and chest radiograph may be carried out in all patients of granuloma with malignancies to pick up smoldering TB cases.
One case of colonic adenocarcinoma in our study had coexisting Crohn’s disease, which is a well-known risk factor for adenocarcinoma.15 SS is another described risk factor in some studies.2,9,16 However, we couldn’t elicit a prior history of SS in any of our cases.
Based on histomorphology, granulomas were classified as necrotizing (TB like) and nonnecrotizing ones (sarcoid-like). In our study, out of the total 35 cases, 22 cases (62.9%) were nonnecrotizing sarcoid-like granulomas, while 13 cases (37.1%) were necrotizing tuberculoid-like granulomas. This is in contrast to a study on lung cancers where necrotizing granulomas were found more frequently with 8 of 19 patients (42%), whereas 6 (31.6%) had nonnecrotizing granulomas.6
Granulomas in TB have been reported to be caseating in 58.7%, non caseating in 23.8% and atypical in 17.5% cases in a previously conducted autopsy study;17 however, in the present study both the AFB positive cases were necrotizing granulomas. Similarly, there have been case reports and studies with ZN stain positivity in necrotizing granulomas.
One study recommended that in cases of ZN positivity, a mastectomy should be performed for operable breast cancer followed by 18 months of anti- TB therapy. To avoid the effect of immunosuppression, chemotherapy is recommended after 4 weeks of anti-TB therapy.18 Hence, treatment guidelines are amenable to modifications in cases of AFB-positive granulomas associated with malignancies.
In our study, out of the total 35 cases, 11 cases (31.4%) had a presence of granuloma in the primary tumor with or without the involvement of lymph nodes and were negative for AFB. Similarly, Dagaonkar et al. found 19 out of 127 cases (14.9%) of lung carcinoma with the presence of granuloma in the primary tumor and were negative for AFB.6 Also, studies conducted by Alalshee et al., Daroca PJ. and Bässler R, Birke F. observed two, three, and five such cases, respectively, of breast carcinoma with primary stromal granulomas.4,19,20
In our study, out of the total 35 cases, 24 cases (68.6%) had a presence of granulomas in draining lymph nodes, of which 5 cases (20.8%) had tumor metastatic deposits, while 19 cases (79.2%) were negative for metastasis. In another study, of all the consecutive lymph node dissections done for cancer patients during one-and-a-half years, 27 cases showed granulomatous inflammation in lymph nodes, of which 2 cases (7.4%) had metastatic deposits.3
In a study done by Krvavac A et al. of 40 lung carcinoma patients, 3 (7.5%) had nodal granulomas without metastatic deposits.21
Several queries, therefore need to be addressed regarding this interesting observation, especially whether a history of prior systemic TB or sarcoidosis needs to be evaluated in all patients having cancer. The presence of necrotizing versus nonnecrotizing granulomas should be further evaluated using ancillary tests like ZN stain for AFB, Polymerase chain reaction (PCR) testing, or Gene-Expert testing. Whether these findings could have any implications in the treatment modifications in cancer patients need to be evaluated.
Some of the older reports have also shown an association of epithelioid granulomas with tumor metastasis in the lymph nodes. A close scrutiny of such granulomas is therefore necessary to avoid underdiagnosis of the metastatic disease. However, it may not be easy to identify the tumor cells in the lymph node, and immunostaining with cytokeratin may be required to rule out occult metastasis, as recommended in some studies.3,4
Pathologists often describe the granulomas in malignancies, but mostly, they are neither specifically mentioned nor elaborated on in the final diagnosis. This is important because clinicians focus on the final pathologic diagnosis, and many microscopic descriptions are not read. This synchronous occurrence also leads to a state of confusion for the treating physician.22 Conscientious data recording in routine synoptic reporting formats for all cases of granulomas coexisting with malignancies may prove an exemplar for larger model multisystemic studies in the future. A large series on this phenomenon may perhaps help to identify the true incidence and prognostic importance of granulomas in draining lymph nodes of a carcinoma.
CONCLUSION
Granulomatous reactions in the stroma usually indicate a T-cell-mediated immunological response to the antigens present on the cell surface. There are only a handful of multisystemic studies on this topic available in English literature. With cases of TB showing both caseating and noncaseating granulomas and lack of a confirmatory gold standard test available for TB, a careful search for AFB on routine ZN stain should be carried out in all the cases of granulomas with or without necrosis. Other ancillary tests such as TB culture, molecular-based PCR, Gene Expert, and regular clinical examination for an increase in the size of the lymph node, production of sputum, etc., should be carried out for all patients with granulomas in malignancies, especially in countries endemic for TB. Presence of occult metastatic tumor cells within necrotic granulomas in the draining regional group of nodes should be identified by immunohistochemistry wherever possible. Granuloma characteristics like necrotizing/nonnecrotizing, confluent/discrete, and tumor deposit present/absent; prior history of systemic/local illnesses (like SS, TB, fungal infections, Inflammatory Bowel Disease (IBD), Chemotherapy (CT)/Radiotherapy (RT)) should be documented in all cases with dual granulomas as well as malignancies.
Acknowledgements
I am grateful to all of those with whom I worked during this study for their knowledge and support.
Ethical approval
The authors declare that they have taken the Institutional Ethics Committee approval and the approval number is IEC/RMCH/86/2022/Aug.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Granulomatous inflammation in lymph nodes draining cancer: A coincidence or a significant association! Int J Med Med Sci. 2009;1:013-6.
- [Google Scholar]
- Interpretation of granulomatous lesions in malignancy. Acta Oncol. 1992;31:85-9.
- [CrossRef] [PubMed] [Google Scholar]
- Granulomatous inflammation in lymph nodes draining cancer: Significant association or just coincidence-diagnostic dilemma. J Diagn Pathol Oncol. 2018;3:295-8.
- [Google Scholar]
- Granulomatous reaction associated with breast carcinoma: A report of two cases. Saudi J Med Med Sci. 2014;2:120-2.
- [Google Scholar]
- Granuloma after breast conserving surgery-a report of three cases. J Surg Case Rep. 2021;6:1-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Significance of coexistent granulomatous inflammation and lung cancer. J Clin Pathol. 2017;70:337-41.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Granulomatous response with breast cancer: A case report. Iran J Pathol. 2016;11:171-5.
- [PubMed] [PubMed Central] [Google Scholar]
- Malignant tumours and sarcoid reactions in regional lymph nodes. Acta Radiol. 1957;47:381-92.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor-associated granulomas preceding a diagnosis of thoracic sarcoidosis: A retrospective, single-center cohort study. J Clin Med. 2021;10:4151.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Papillary carcinoma of thyroid with an unusual coexistence of metastatic deposits and tuberculosis in the cervical lymph nodes. Med J DY Patil Univ. 2014;7:59-61.
- [Google Scholar]
- Necrobiotic palisading granulomas associated with breast carcinoma. J Clin Pathol. 2005;58:1290-3.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Immunopathogenesis of delayed type hypersensitivity. Microsc Res Technol. 2001;15:241-5.
- [CrossRef] [PubMed] [Google Scholar]
- Granulomas in association with neoplasm: A reaction or a different primary process? J Postgrad Med. 2009;55:234-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative Study of GeneXpert with ZN stain and culture in samples of suspected pulmonary tuberculosis. J Clin of Diagn Res. 2016;10:DC09-DC12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Colorectal carcinoma in patients with Crohn’s disease. Gastroenterology. 1985;89:398-407.
- [CrossRef] [PubMed] [Google Scholar]
- Sarcoidosis and cancer: A complex relationship. Front Med. 2020;7:594118.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Liver involvement in tuberculosis-an autopsy study. Trop Gastroenterol. 2006;27:69-74.
- [PubMed] [Google Scholar]
- Coexistence of carcinoma and tuberculosis in one breast. World J Surg Oncol. 2008;6:29.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Medullary carcinoma of the breast with granulomatous stroma. Hum Pathol. 1987;18:761-3.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathology of tumour associated sarcoid-like stromal reaction in breast cancer. An analysis of 5 cases with immunohistochemical investigations. Virchows Arch A Pathol Anat Histopathol. 1988;412:231-9.
- [CrossRef] [PubMed Central] [Google Scholar]
- The concurrence of granulomatous inflammation in intrathoracic lymph nodes with regional metastasis from primary lung cancer in surgically resected specimens. Adv Respir Med. 2018;86:215-9.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculosis and metastatic carcinoma coexistence in axillary lymph node: A case report. World J Surg Oncol. 2003;1:3.
- [CrossRef] [PubMed] [Google Scholar]





