Translate this page into:
Pancreatic Incidentalomas: Review and Current Management Recommendations
Address for correspondence Binit Sureka, MD, DNB, MBA, Department of Diagnostic and Interventional Radiology, All India Institute of Medical Sciences, Basni Industrial Area, MIA 2nd Phase, Basni, Jodhpur 342005, Rajasthan, India (e-mail: binitsurekapgi@gmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
There has been significant increase in the detection of incidental pancreatic lesions due to widespread use of cross-sectional imaging like computed tomography and magnetic resonance imaging supplemented with improvements in imaging resolution. Hence, accurate diagnosis (benign, borderline, or malignant lesion) and adequate follow-up is advised for these incidentally detected pancreatic lesions. In this article, we would review the various pancreatic parenchymal (cystic or solid) and ductal lesions (congenital or pathological), discuss the algorithmic approach in management of incidental pancreatic lesions, and highlight the key imaging features for accurate diagnosis.
Keywords
duct
incidentaloma
pancreas
pancreatic cyst
Introduction
The term “pancreatic incidentaloma” (PI) refers to those lesions in the pancreas that are diagnosed incidentally when obtaining an imaging study of the abdomen not intended to look for a pancreatic pathological process.1 This phenomenon is not new to the pancreas and has been quite often observed in other organs such as adrenal glands, thyroid, parathyroid, pituitary, liver, prostate, and kidneys. The first case of PI was described by Kostiuk in 2001.2 Incidental pancreatic cysts are frequently encountered now due to advancements and easy availability of imaging. The reported incidence of incidental cystic pancreatic lesions varies, depending on the imaging technique used. In computed tomography (CT), the prevalence varies from 0.5 to 3%, whereas in magnetic resonance imaging (MRI), this prevalence increases to 18 to 19.6%.3,4 In a postmortem study by Kimura et al, cysts less than 1 cm in size were detected in 24% of cases.5 Recent published studies have shown that the incidence of incidental PI is rising.6
When a PI is encountered, the first aim is to classify whether it is a pancreatic parenchymal or ductal lesion. PIs in the pancreatic parenchyma can be either cystic or solid. Incidental lesions in the main pancreatic duct (MPD) can be congenital variations or a pathological process involving the MPD. The second aim is to further classify the lesion into benign or malignant. Various cystic and solid incidentalomas in the pancreatic parenchyma are listed in ►Table 1.7
| Exocrine | Infections |
| Benign SCN MCN IPMN Mature cystic teratoma Borderline Mucinous cystic tumor with moderate dysplasia Intraductal papillary mucinous tumor with moderate dysplasia SPN Malignant Ductal adenocarcinoma Osteoclastlike giant cell tumor Serous cystadenocarcinoma Mucinous cystadenocarcinoma IPMN Acinar cell carcinoma Pancreatoblastoma Solid pseudopapillary carcinoma Ampullary adenocarcinoma |
Mycobacterium avium complex Mycobacterium tuberculosis Rare and atypical fungal and viral infections |
| Mesenchymal tumors | |
| Kaposi's sarcoma Lipoma Lymphangioma Pancreatic Castleman's disease Pancreatic hamartoma Schwannoma Teratoma |
|
| Metastases | |
| Colon, breast, lung, lymphoma, melanoma, renal cell carcinoma | |
| Endocrine | Nonislet cell tumors |
| Gastrinoma Glucagonoma GRF-secreting tumor Insulinoma PP-secreting tumor Somatostatinoma VIPoma Serotoninoma |
Adenosquamous carcinoma Anaplastic tumors Colloid carcinoma Granulocytic sarcoma Leukemia Lymphoma Primitive neuroectodermal tumor |
| Cystic lesions | Inflammatory |
| Benign pancreatic cysts Dysontogenic cysts Hydatid cyst Cysticercosis LECs Pancreatic dermoid cysts Retention pancreatic cysts |
Eosinophilic pancreatitis Focal pancreatitis Inflammatory myofibroblastic tumor Lymphoid hyperplasia Wegener's disease Xanthogranulomatous pancreatitis |
| Congenital | |
| Congenital cyst Epidermoid cyst in IPAS |
Abbreviations: GRF, growth hormone releasing factor; IPAS, intrapancreatic accessory spleen; IPMN, intraductal papillary mucinous adenoma; LEC, lymphoepithelial cyst; MCN, mucinous cystadenoma; PP, pancreatic polypeptide; SCN, serous cystadenoma; SPN, solid pseudopapillary tumor; VIPoma, pancreatic neuroendocrine tumor that secretes vasoactive intestinal peptide.
Incidental Cystic Pancreatic Tumors
Most incidental cystic pancreatic lesions are benign.8,9 The first step is to differentiate these cystic lesions from pancreatic pseudocysts. Serous cystadenoma, mucinous cystic lesions, and intraductal papillary mucinous neoplasms (IPMNs) account for more than 90% of primary cystic pancreatic tumors.10 Most of the patients are asymptomatic at the time of presentation. Symptomatic patients may present with abdominal pain, jaundice, weight loss, and/or recurrent episodes of pancreatitis. Morphological classification of cystic lesions of the pancreas is given in ►Table 2.11
| Unilocular | Microcystic |
| Pseudocyst Cystic neuroendocrine tumor Unilocular serous cystadenoma Unilocular mucinous cystadenoma Retention cyst Developmental cyst Epithelial cyst Lymphoepithelial cyst Epidermoid cyst in intrapancreatic accessory spleen Endometrial cyst Infectious cyst |
Serous cystadenoma Microcystic variant of ductal adenocarcinoma (very rare) |
| Macrocystic | Cystic transformation of the pancreas |
| Mucinous cystadenoma BD-IPMN Oligocystic serous cystadenoma Lymphangioma Lymphoepithelial cyst Infectious cyst Duplication cyst Mesothelial cyst |
Dysontogenetic cyst Cystic fibrosis Disseminated serous cystadenoma Congenital syndromes such as von Hippel–Lindau's disease, polycystic kidney disease, Ivemark's syndrome, trisomy 13 or 15, Meckel–Gruber's syndrome |
| Cyst with ductal communication | Multifocal |
| IPMN Collections postpancreatitis as a part of disconnected duct syndrome Retention cyst/squamoid cyst |
BD-IPMN Pseudocysts Serous cystadenoma Neuroendocrine tumor Developmental cyst Epithelial cyst |
| Solid cystic | |
| SPN Pancreatoblastoma Cystic metastasis Cystic degeneration in solid tumors Malignant transformation in cystic tumors Metastases Hemorrhagic pseudocyst |
|
Abbreviations: BD-IPMN, branch-duct intraductal papillary mucinous neoplasm; IPMN, intraductal papillary mucinous neoplasm; SPN, solid pseudopapillary tumor.
Unilocular Cysts
Pancreatic pseudocysts are the most commonly encountered unilocular cysts. Others include mucinous and serous cystadenoma, lymphoepithelial cysts, retention cyst, developmental cyst, epidermoid cyst in intrapancreatic spleen, endometrial cyst, and cystic neuroendocrine tumor or an infectious cyst (►Figs. 1 and 2). A unilocular lesion in a patient with a clinical history of pancreatitis is almost always a pseudocyst. A lobulated unilocular cyst located in the head of the pancreas should raise the suspicion of a serous cystadenoma.

- A 43-year-old-female with incidentally detected lymphoepithelial cyst postcholecystectomy in the pancreas. (A) Axial T1-weighted magnetic resonance imaging showing unilocular cystic lesion, which is mildly hyperintense in the head of the pancreas (B) and hyperintense on T2-weighted images (C), showing bright signal on diffusion-weighted imaging (D) with no enhancement on T1-weighted postcontrast gradient echo images.
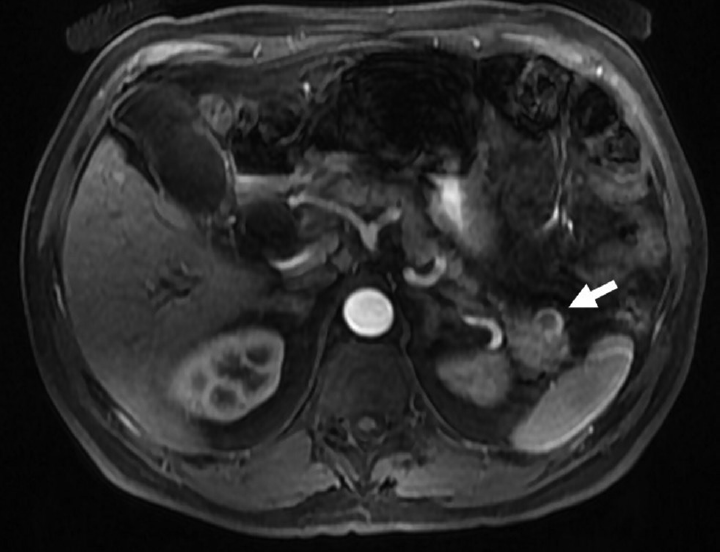
- A 60-year-old male with incidental cystic pancreatic neuroendocrine tumor. Axial postcontrast T1-weighted image showing incidental unilocular cystic lesion (arrow) in the tail of the pancreas, showing peripheral arterial enhancement suggestive of cystic neuroendocrine tumor.
Microcystic Lesions
The most important differential diagnosis of a microcystic lesion in the pancreas is serous cystadenoma. It usually demonstrates a polycystic or microcystic pattern consisting of cysts up to 2 cm in size. They are usually lobulated. The septa and wall may show enhancement (►Fig. 3). A stellate pattern of calcification is visible in 30% of the patients and is considered a characteristic of a serous cystadenoma.12 A rare differential diagnosis of a microcystic lesion in the pancreas is a microcystic variant of ductal adenocarcinoma.13
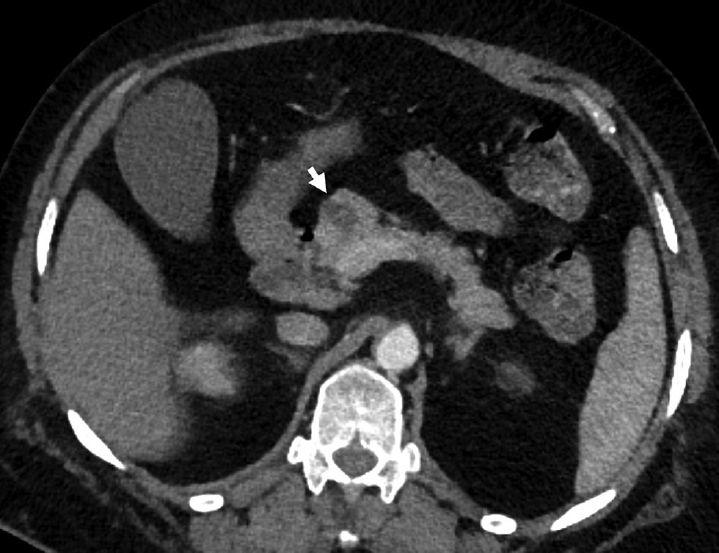
- A 68-year-old-female with incidental serous cystadenoma. Axial contrast-enhanced computed tomography image showing polycystic lesion in the head of the pancreas (arrow) with thin internal septations in a case of serous cystadenoma.
Macrocystic Lesions
Mucinous cystic neoplasms (cystadenomas) and branch-duct (BD) IPMNs usually present as macrocystic lesions. Mucinous cystadenomas mainly involve the body and tail of the pancreas and do not communicate with the MPD. It is important to differentiate serous from mucinous cystic neoplasm as surgery is the treatment of choice in mucinous cystic neoplasm.14 A peripheral eggshell calcification is highly suggestive of a potentially malignant mucinous cystic neoplasm.15 Other differential diagnoses of macrocystic lesions in the pancreas are lymphangiomas, lymphoepithelial cysts, infectious cysts, mesothelial cysts, and duplication cysts.
Cyst with Solid Component (Solid Cystic)
Tumors with this morphology are solid pseudopapillary neoplasms, pancreatoblastomas, cystic metastases, cystic degeneration in solid tumors, malignant transformation in cystic tumors, and hemorrhagic pseudocysts. Cysts with a solid component can be unilocular or multilocular and may or may not have ductal communication. Therefore, true cystic tumors with solid component as well as solid pancreatic neoplasms with a cystic component or cystic degeneration are included in this category. Most tumors in this category are malignant and should be surgically treated. MR cholangiopancreatography (MRCP) is superior to single-section helical CT to characterize these tumors.16,17
Cystic Transformation of the Pancreas
Cystic transformation of the pancreas is seen in dysontogenetic cyst, cystic fibrosis, disseminated variant of serous cystadenoma, congenital syndromes such as von Hippel–Lindau's disease (VHL), autosomal dominant polycystic kidney disease (ADPKD), Ivemark's syndrome, trisomy 13 or 15, Meckel–Gruber's syndrome, and so on.18,19 Polycystic disease of the pancreas is also known as dysontogenetic cysts or congenital cysts of the pancreas. It is a very rare entity that may occur as a solitary cyst, polycystic disease in association with renal cysts, and liver, central nervous system, or retinal abnormalities. Pancreatic involvement in VHL is in the form of simple cysts (71%), serous cystadenomas (15%), pancreatic neuroendocrine tumors (pNETs; 10%) and rarely cystic replacement of the entire pancreas.20,21 Kim et al22 have shown that pancreatic cysts are five times more prevalent in patients with ADPKD with PKD2 mutation than in patients with PKD1 mutation. PKD1 has a more aggressive disease course, with an earlier age of symptom onset, end-stage renal disease, and death. Thus, the potential to discriminate PKD1 from PKD2 on MRI has important prognostic implications. MRI identification of pancreatic cysts in ADPKD significantly increases the likelihood that a PKD2 mutation is present.23,24
Cyst with Ductal Communication
Tumors included in this subgroup are IPMNs, collections postpancreatitis as a part of disconnected duct syndrome, and retention cysts. IPMNs are more common in elderly males in the sixth to seventh decade of life. Three types of IPMNs may be observed: main duct, BD, and mixed variant.25 Retention cysts are cystic dilatation of the pancreatic duct. It may or may not be associated with an obstructive cause such as calculi, stricture, mucin plugs, and tumors.26
Multifocal Cystic Lesions
BD-IPMN, pseudocysts, serous cystadenomas, neuroendocrine tumors, developmental cysts, and epithelial cysts can be multifocal and should be considered in the differential diagnoses of multifocal cystic lesions of the pancreas.11
Incidental Solid Pancreatic Tumors
The incidence of benign disease in solid pancreatic tumors suspicious of cancer ranges from 6 to 21%. Chronic pancreatitis presenting as inflammatory pancreatic mass accounts for almost 70% of the benign lesions,27 with alcoholic pancreatitis being the most common cause (60%) with autoimmune pancreatitis (AIP) in up to 11% of the patients.28,29
Pancreatic Adenocarcinoma
The most frequent solid lesion in the pancreas is pancreatic ductal adenocarcinoma (PDAC). Symptomatic patients present with advanced disease at the time of diagnosis (extensive local disease in ~40% and metastases in 40–55%), leaving less than 20% of patients as candidates for potentially curative resection.30,31 The earliest imaging finding of a PDAC before a mass becomes apparent is pancreatic ductal dilatation or pancreatic duct cutoff. On imaging, pancreatic carcinomas (PCs) are hypovascular, hypoenhancing lesions when compared with the surrounding pancreatic parenchyma. On MRI, most PDACs are hypointense on unenhanced T1-weighted sequences when compared with the surrounding pancreas and are hypointense or isointense on T2-weighted images.32,33 The sensitivity and specificity of fluoro-2-deoxy-d-glucose–positron emission tomography (FDG-PET) for the diagnosis of PC in patients with normal blood glucose levels range from 85 to 100% and 67 to 99%, respectively. Combination of PET and CT may offer a better accuracy.34 Serum tumor markers can be helpful in differentiating benign from malignant pancreatic masses. The addition of other tumor markers such as Ca-125 does not increase the diagnostic accuracy of CA19-9 and is the gold standard marker for PDAC with sensitivity and specificity as high as 87 and 98%, respectively.35
Pancreatic Neuroendocrine Tumors
Over the last decade, the wide use of imaging technology has led to the rising incidence of pNETs. pNETs are rare and account for 2 to 4% of all pancreatic neoplasms, with an incidence of 1.5 in 100,000.36,37 In recent years, the detection of incidentally nonfunctioning pNETs (NF-pNETs) has rapidly increased due to the widespread use of endoscopic and cross-sectional imaging. Nearly 60% secrete one or more biologically active peptides, resulting in clinical syndromes. The most frequent functioning tumors are insulinomas, gastrinomas, glucagonomas, VIPomas (a pancreatic neuroendocrine tumor that secretes vasoactive intestinal peptide), and somatostatinomas. Between 30 and 40% of pNETs are nonfunctioning, and this is more likely to be discovered incidentally when symptoms due to the presence of the mass are not yet obvious.38
On CT scan, most pNETs are isodense or moderately hypodense masses showing good arterial enhancement (►Fig. 4). Calcification, necrosis, and cystic degeneration seem to be more common in large nonfunctioning tumors or with malignant transformation. MRI has a diagnostic sensitivity of 78 to 91%, which is equivalent to that of dynamic CT.39,40 On MRI, pNETs show low signal intensity on T1-weighted images and high signal intensity on T2-weighted images.41,42 The most important imaging differential diagnosis is intrapancreatic spleen, which shows enhancement characteristics similar to those of splenic parenchyma (►Fig. 5).
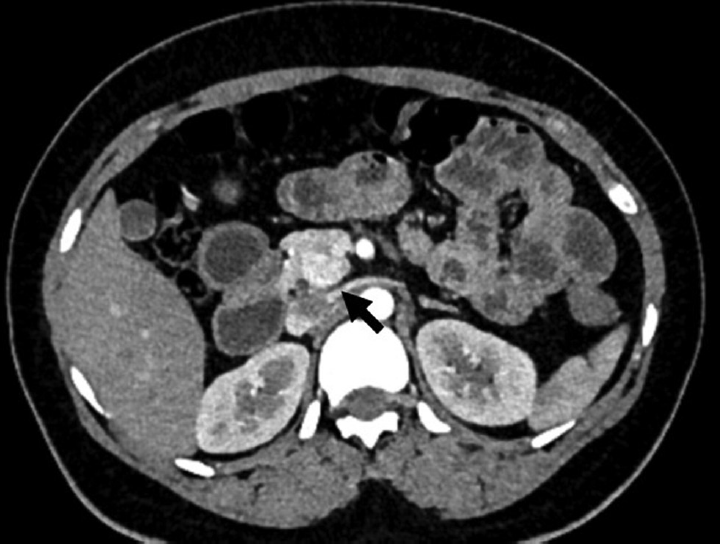
- A 27-year-old female with incidental pancreatic neuroendocrine tumor. Axial contrast-enhanced computed tomography image showing incidental well-defined hypervascular lesion (arrow) in head uncinate of the pancreas.
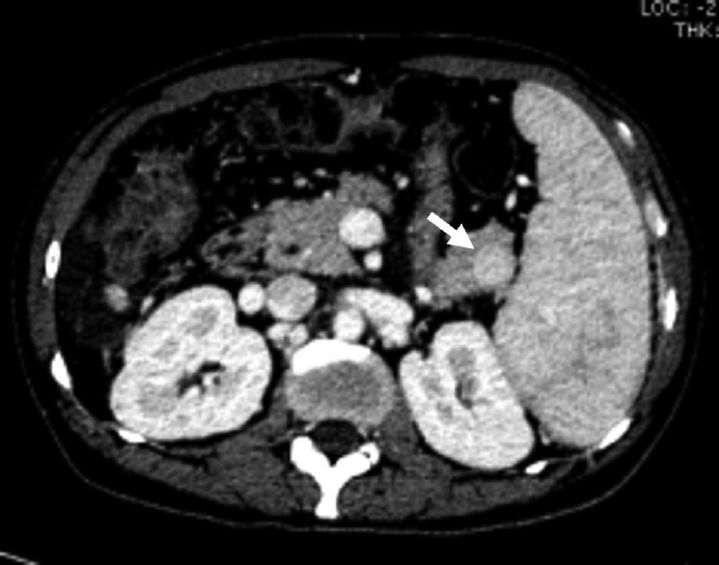
- A 45-year-old-male with intrapancreatic spleen. Axial contrast-enhanced computed tomography image showing solid enhancing lesion (arrow) in the tail of the pancreas with enhancement similar to splenic parenchyma suggestive of intrapancreatic spleen.
Inflammatory Pancreatic Mass
The combination of acute pancreatitis and cancer is unusual. Pancreatic cancer represents 1 to 2% of acute pancreatitis etiologies, and only 3% of cancers manifest as acute pancreatitis.43 The risk of developing pancreatic cancer in patients with chronic pancreatitis is around 15 times higher than in the average population.44 Dilatation of the pancreatic duct, and double-duct sign with both biliary and pancreatic obstruction or interruption of the pancreatic duct are unusual and should lead to considering an underlying carcinoma. Sheathing of the celiac trunk and/or mesenteric artery is seen in 30 to 60% of CT scans of adenocarcinoma.45 But this can also be seen in AIP or IgG4 (immunoglobulin G4 related) conditions with extrapancreatic lesions in sclerosing mesenteritis and retroperitoneal fibrosis.46
Adenocarcinoma developing in the background of chronic pancreatitis is difficult to detect on imaging. In the context of chronic pancreatitis, calcifications displaced by the mass is a pointer suggesting a coexisting PC.47,48 Nearly 10% of presumed PCs that are surgically operated have been composed of pseudotumoral forms of pancreatitis, nearly half of which are thought to be focal forms of AIP, involving mainly the head.49,50 Certain imaging criteria are helpful in diagnosing AIP, such as both early and delayed homogeneous enhancement of the lesion close to that of normal parenchyma, peripheral pseudocapsule, a duct visible in the mass with an hourglass stenosis, absence of upstream atrophy or marked dilatation of the pancreatic duct (<4 mm), multifocal involvement, absence of vascular involvement, and presence of extrapancreatic manifestations.51
Incidental Congenital Main Pancreatic Duct Anomalies
Congenital anomalies and normal variants of the pancreas and pancreatic duct may not be detected until adulthood and are often discovered as an incidental finding in asymptomatic patients. These anomalies are considered and detected only when patients present with idiopathic pancreatitis. MRCP is the modality of choice nowadays for the assessment of congenital pancreatic anomalies since it depicts ductal anatomy rapidly and noninvasively. Anatomic variations and developmental anomalies of the pancreas and pancreatic duct include variations of the course of the pancreatic duct (descending, sigmoid, vertical, and loop-shaped course), variation of the configuration of the pancreatic duct (bifid configuration with dominant duct of Wirsung [60%], dominant duct of Santorini without divisum [1%], absent duct of Santorini, and ansa pancreatica), duplication anomalies, anomalous pancreaticobiliary ductal junction, pancreas divisum (4–14%), annular pancreas, ectopic pancreas, and pancreatic agenesis and hypoplasia of the dorsal pancreas and accessory pancreatic lobe.52,53
Incidental Pathological Processes Involving the MPD
Genetic mutation associated pancreatitis (GMAP) also sometimes referred to as idiopathic painless chronic pancreatitis can present as an incidental ductal disease process while imaging for symptoms not related to the pancreas. Several gene mutations associated with chronic pancreatitis have been identified, with the most frequent involving the CFTR (cystic fibrosis transmembrane regulator) gene, the SPINK1 (serine protease inhibitor, Kazal type 1) gene, and the PRSS1 (cationic trypsinogen) gene. According to some authors, the patients with pancreatitis associated with one of these gene mutations show onset at a younger age than those with pancreatitis related to other factors, even though the diagnosis is often late compared with the appearance of symptoms. Accurate diagnosis of GMAP is important for a careful follow-up of these patients, as the risk of developing pancreatic adenocarcinoma is higher in this group than in the normal population or in patients affected by chronic pancreatitis not associated with gene mutations. On imaging, typical bull's eye pattern of stones, with a dense peripheral rim and a noncalcified radiolucent central core with stones greater than 15 mm in size, is seen.54,55
pNETs expressing serotonin (carcinoid tumors) account for a small portion of neuroendocrine tumors.56 Segmental changes in the pancreatic duct are being increasingly encountered as patients undergo abdominal imaging for evaluation of a variety of symptoms. The two most common causes of segmental pancreatic duct dilatation and pancreatic atrophy are chronic pancreatitis and malignant neoplasms such as PDAC. In rare instances, small serotonin secretin neuroendocrine tumors (serotoninoma) can induce fibrogenesis due to production of 5-hydroxyindoleacetic acid and serotonin, leading to obstruction of the pancreatic duct.57,58 These tumors are often detected incidentally while imaging patients for symptoms other than pancreatic etiology.
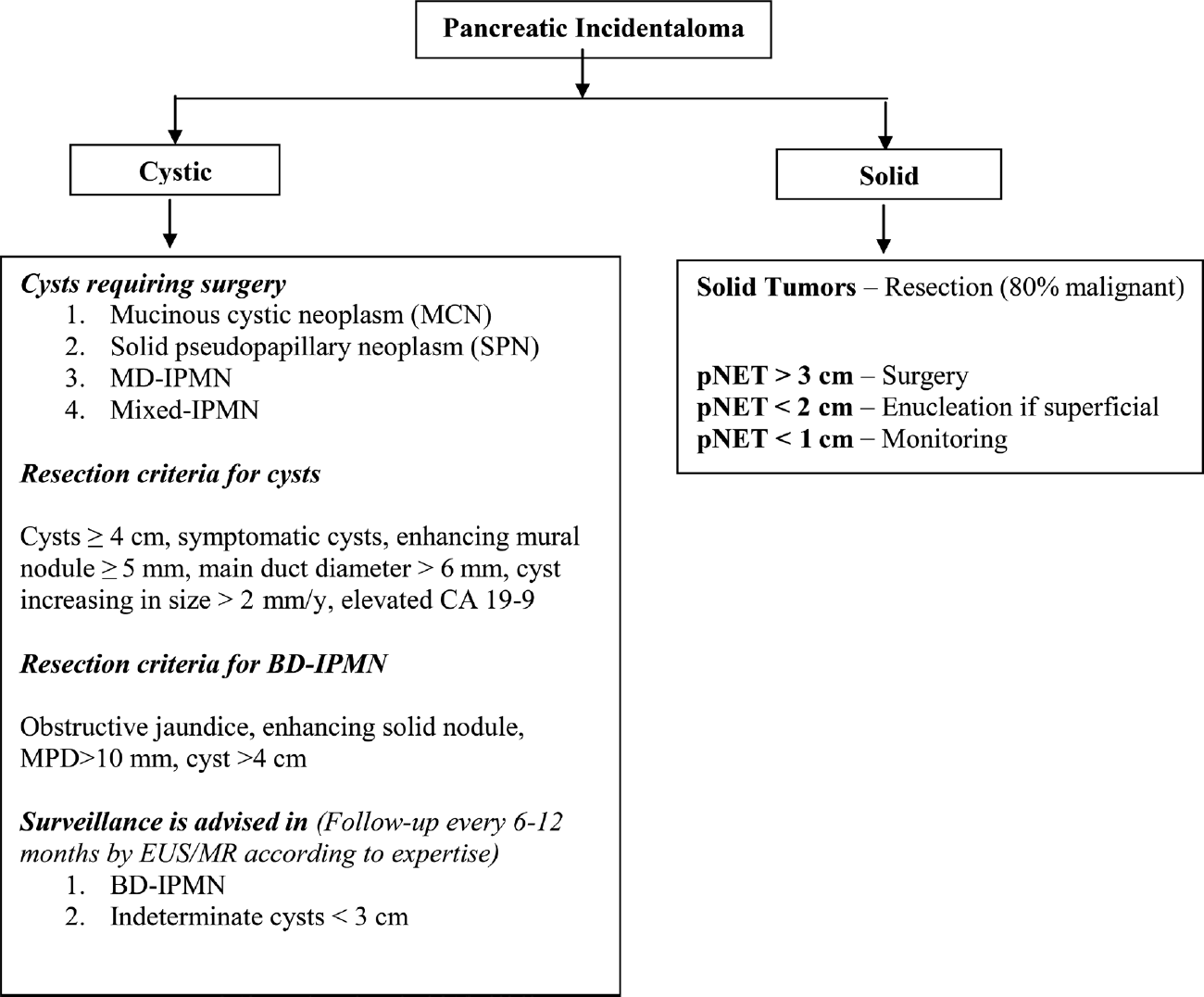
Management of Incidental Pancreatic Lesions
Most guidelines reach the consensus that the presence of a potentially resectable solid pancreatic mass in a CT scan or endoscopic ultrasound (US) in an otherwise healthy patient, with no clinical or biochemical characteristics suggesting a benign condition, should prompt surgical treatment.27 Management of incidentally detected pNETs is a debatable topic. Indications for surgery in pNETs are functioning pNET and NF-pNETs > 2 cm. pNET < 1 cm can be kept on monitoring, and pNETs of 1 to 2 cm should be considered for enucleation if superficial in location.59-61
Various published guidelines for the management of cystic lesions recommend resection of potentially malignant tumors such as mucinous cystadenomas, solid pseudopapillary tumors, and main and mixed type of intraductal papillary mucinous neoplasms and observe benign lesions such as serous cystadenoma (< 4 cm) and BD IPMN.62-65 Close follow-up and surgical consideration are recommended in cases with worrisome features (symptomatic, cytology suspicious for malignancy, enhancing mural nodule < 5 mm, MPD > 6 mm, cyst size increasing > 2 mm/year, elevated CA19-9).62-65 The International Association of Pancreatology recommends surveillance for simple cysts < 3 cm in size. Patients with cysts < 1 cm are imaged at an interval of 2 to 3 years (CT/MRI), those with cysts 1 to 2 cm in diameter at an interval of 1 year (CT/MRI), and those with cysts of size 2 to 3 cm at an interval of 3 to 6 months (preferably endoscopic US).64 An algorithmic approach to the management of PIs is illustrated in ►Fig. 6.
Conclusion
PIs are increasingly encountered by the radiologists today and are worrisome. These can be cystic or solid. An incidentally discovered pancreatic cystic lesion without any evidence of worrisome features and of < 2 cm is highly unlikely to cause morbidity or mortality. Around half of these lesions may eventually grow to be larger than 2 cm; therefore, adequate follow-up is advised. Solid PIs > 1 cm are potentially considered malignant, unless proven otherwise, and resection or enucleation is recommended. Key practical tips for the radiologists when dealing with PIs are given in ►Table 3.
| CT using pancreatic protocol is the imaging of choice for solid lesions MRI is the modality of choice in characterizing and for follow-up of cystic lesions EUS and fluid aspirate analysis are used for indeterminate lesions Solid pancreatic incidentalomas are considered malignant unless until proven otherwise High amylase and CEA levels in fluid suggest pseudocyst and mucinous neoplasms, respectively Simple cysts up to 3 cm in size with no worrisome features can be safely monitored Surgery is considered for MCN, SPN, MD-IPMN, mixed IPMN Surgery is considered for cysts > 3 cm and serous cystadenoma > 4 cm Solid lesions up to 2 cm in size are likely to be malignant and will require surgical intervention Small pNETs (<1 cm) are simply monitored Larger pNETs (>2 cm) lesions are treated by enucleation or resection Generally, pancreatic incidentalomas have a better prognosis than symptomatic lesions |
Abbreviations: CEA, carcinoembyronic antigen; CT, computed tomography; EUS, endoscopic ultrasound; IPMN, intraductal papillary mucinous neoplasm; MCN, mucinous cystadenoma; MD-IPMN, main-duct intraductal papillary mucinous neoplasm; MRI, magnetic resonance imaging; pNETs, pancreatic neuroendocrine tumors; SPN, solid pseudopapillary tumor.
Conflict of Interest
None declared.
References
- Pancreatic Incidentaloma [Internet] (updated 2013 June 06 Available at: https://www.sages.org/wiki/pancreatic-incidentaloma (accessed ) (accessed )
- [Google Scholar]
- High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8(09):806-811.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223(02):547-553.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995;18(03):197-206.
- [CrossRef] [PubMed] [Google Scholar]
- Periampullary and pancreatic incidentaloma: a single institution's experience with an increasingly common diagnosis. Ann Surg. 2006;243(05):673-680.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic incidentaloma. In: Hubbard JGH, Inabnet WB, Lo CY, eds. Endocrine Surgery: Principles and Practice (Springer Specialist Surgery Series) (1st). London: Springer; 2009.
- [Google Scholar]
- Incidental pancreatic cysts: clinico-pathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138(04):427-433.
- [CrossRef] [PubMed] [Google Scholar]
- A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg. 2006;244(04):572-582.
- [Google Scholar]
- Cystic lesions of the pancreas: selection criteria for operative and nonoperative management in 209 patients. J Gastrointest Surg. 2003;7(08):970-977.
- [CrossRef] [PubMed] [Google Scholar]
- JOP. 2016;17:452-465.
- CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? Am J Roentgenol. 2000;175(01):99-103.
- [CrossRef] [PubMed] [Google Scholar]
- Large duct type invasive adenocarcinoma of the pancreas with microcystic and papillary patterns: a potential microscopic mimic of non-invasive ductal neoplasia. Mod Pathol. 2012;25(03):439-448.
- [CrossRef] [PubMed] [Google Scholar]
- An aggressive resectional approach to cystic neoplasms of the pancreas. Am J Surg. 1999;178(04):269-274.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic cystic neoplasms. Radiol Clin North Am. 1989;27(01):163-176.
- [CrossRef] [PubMed] [Google Scholar]
- Intraductal papillary mucinous tumors of the pancreas: helical CT with histopathologic correlation. Radiology. 2000;217(03):757-764.
- [CrossRef] [PubMed] [Google Scholar]
- Cystic lesions and neoplasms of the pancreas . The features are becoming clearer. Pancreatology. 2001;1(06):648-655.
- [CrossRef] [PubMed] [Google Scholar]
- Polycystic disease of the pancreas (dysontogenetic cysts): report of a case with partial pancreatectomy. Ann Surg. 1937;106(01):49-53.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic involvement in von Hippel-Lindau disease . The Groupe Francophone d'Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119(04):1087-1095.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic cyst development: insights from von Hippel-Lindau disease. Cilia. 2013;2(01):3.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic cysts in autosomal dominant polycystic kidney disease: prevalence and association with PKD2 gene mutations. Radiology. 2016;280(03):762-770.
- [CrossRef] [PubMed] [Google Scholar]
- Conference Participants . Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015;88(01):17-27.
- [CrossRef] [PubMed] [Google Scholar]
- The utilization of imaging features in the management of intraductal papillary mucinous neoplasms. Gastroenterol Res Pract. 2014;2014:765451.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic retention cyst: multi-modality imaging findings and review of the literature. Abdom Imaging. 2013;38(04):818-826.
- [CrossRef] [PubMed] [Google Scholar]
- Management of pancreatic masses. Pancreas. 2005;31(03):203-217.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic cancer: cost-effectiveness of imaging technologies for assessing resectability. Radiology. 2001;221(01):93-106.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic imaging: current and emerging technologies. Pancreas. 2006;33(03):211-220.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic imaging for pancreatic cancer: computed tomography, magnetic resonance imaging, and positron emission tomography. Pancreas. 2004;28(03):273-278.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective evaluation of pancreatic tumors: accuracy of MR imaging with MR cholangiopancreatography and MR angiography. Radiology. 2002;224(01):34-41.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of positron emission tomography and CT in evaluating pancreatic tumors: technical and clinical implications. Am J Roentgenol. 2003;181(02):387-393.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer antigens 19-9 and 125 in the differential diagnosis of pancreatic mass lesions. Arch Surg. 2006;141(10):968-973.
- [CrossRef] [PubMed] [Google Scholar]
- One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063-3072.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann Oncol. 2008;19(05):903-908.
- [CrossRef] [PubMed] [Google Scholar]
- Islet cell tumors of the pancreas: the medical oncologist's perspective. Surg Clin North Am. 2001;81(03):527-542.
- [CrossRef] [PubMed] [Google Scholar]
- The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev. 2004;25(03):458-511.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging appearances of metastases from neuroendocrine tumours of the pancreas. Br J Radiol. 2001;74(887):1065-1070.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of small, functional islet cell tumors in the pancreas: selection of MR imaging sequences for optimal sensitivity. Radiology. 2000;214(02):483-490.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroendocrine tumors of the pancreas: spectrum of appearances on MRI. J Magn Reson Imaging. 2000;11(02):141-148.
- [CrossRef] [Google Scholar]
- Pancreatitis associated with pancreatic carcinoma: a study of 26 cases. Mayo Clin Proc. 1971;46(03):174-177.
- [Google Scholar]
- Chronic inflammation and pancreatic cancer. Best Pract Res Clin Gastroenterol. 2008;22(01):65-73.
- [CrossRef] [PubMed] [Google Scholar]
- Thickening of the celiac axis and/or superior mesenteric artery: a sign of pancreatic carcinoma on computed tomography. Radiology. 1981;141(02):449-453.
- [CrossRef] [PubMed] [Google Scholar]
- Obliteration of periarterial retropancreatic fat on CT in pancreatitis: an exception to the rule. Am J Roentgenol. 1989;153(01):63-64.
- [CrossRef] [PubMed] [Google Scholar]
- A solid pancreatic mass: tumour or inflammation? Diagn Interv Imaging. 2013;94((07) (08)):741-755.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic mass due to chronic pancreatitis: correlation of CT and MR imaging features with pathologic findings. Am J Roentgenol. 2001;177(02):367-371.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of the focal type of autoimmune pancreatitis in chronic pancreatitis suspected to be pancreatic carcinoma: experience of a single tertiary cancer center. Scand J Gastroenterol. 2008;43(01):110-116.
- [CrossRef] [PubMed] [Google Scholar]
- Autoimmune pancreatitis: CT patterns and their changes after steroid treatment. Radiology. 2008;247(02):435-443.
- [CrossRef] [PubMed] [Google Scholar]
- Pitfalls in avoiding operation for autoimmune pancreatitis. Surgery. 2011;150(05):968-974.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital variants and anomalies of the pancreas and pancreatic duct: imaging by magnetic resonance cholangiopancreaticography and multidetector computed tomography. Korean J Radiol. 2013;14(06):905-913.
- [CrossRef] [PubMed] [Google Scholar]
- Embryology, normal variation, and congenital anomalies of the pancreas. In: Stevenson GW, Freeny PC, Margulis AR, Burhenne HJ, eds. Margulis and Burhenne's Alimentary Tract Radiology (5th). 1994. p. :1039-1051.
- [Google Scholar]
- Bull's-eye pattern of pancreatic-duct stones on multidetector computed tomography and gene-mutation-associated pancreatitis (GMAP) Radiol Med (Torino). 2012;117(08):1275-1286.
- [CrossRef] [PubMed] [Google Scholar]
- Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med. 1998;339(10):653-658.
- [CrossRef] [PubMed] [Google Scholar]
- Serotonin immunoreactivity in pancreatic endocrine neoplasms (carcinoid tumors) Mod Pathol. 1991;4(06):727-732.
- [Google Scholar]
- Fibrosis and carcinoid syndrome: from causation to future therapy. Nat Rev Endocrinol. 2009;5(05):276-283.
- [CrossRef] [PubMed] [Google Scholar]
- Small serotonin-producing neuroendocrine tumor of the pancreas associated with pancreatic duct obstruction. Am J Roentgenol. 2011;197(03):W482-8.
- [CrossRef] [PubMed] [Google Scholar]
- Radical surgery for large-sized slow-growing neuroendocrine tumor of the pancreas. J BUON. 2002;7(04):381-383.
- [Google Scholar]
- Non-functional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg. 2010;14(03):541-548.
- [CrossRef] [PubMed] [Google Scholar]
- Management of cystic and solid pancreatic incidentalomas: a review analysis. J BUON. 2013;18(01):17-24.
- [Google Scholar]
- Follow-up of asymptomatic pancreatic cysts in clinical practice: a vignette questionnaire. Pancreatology. 2016;16(03):416-422.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical Guidelines Committee; American Gastroenterology Association . American Gastroenterological Association Institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148(04):819-822.
- [CrossRef] [PubMed] [Google Scholar]
- Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17(05):738-753.
- [CrossRef] [PubMed] [Google Scholar]
- European Study Group on Cystic Tumours of the Pancreas . European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45(09):703-711.
- [CrossRef] [PubMed] [Google Scholar]