Translate this page into:
Predictors of Poor Response to Salvage Chemotherapy in Relapsed/ Refractory Pediatric Hodgkin Lymphoma- A Retrospective Analysis from Tertiary Cancer Centre in India
Correspondence: Dr. Sameer Bakhshi, Professor of Pediatric Oncology, Department of Medical Oncology, Dr. B. R.A. Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi-110029. Tel: 91-11-26589200 Extn. 5237. Email: sambakh@hotmail.com.
Abstract
Background:
Previous studies identified prognostic factors for survival in relapsed pediatric Hodgkin lymphoma (HL) who received salvage chemotherapy followed by autologous stem cell transplant (ASCT). However, data regarding predictors of poor response to salvage chemotherapy is limited.
Methods:
We conducted retrospective study in all relapsed HL treated from January 2003 to December 2013. Logistic regression analysis was done to identify predictors of response to salvage chemotherapy. Cox regression analysis was done to identify prognostic factors for Freedom from treatment failure (FFTF) and overall survival (OS).
Results:
Forty six patients had relapsed HL. Among 45 patients who received salvage chemotherapy only 34 (73.4%) underwent ASCT. Stage 4 disease (p=0.02) and bulky disease at relapse (p=0.03) were predictors of poor response to salvage chemotherapy. FFTF and OS at 5 yr for entire cohort were 50.1% and 63.3%, respectively, while the same for patients who underwent ASCT were 66.3% and 80.7%, respectively. Among ASCT patients, those who had primary refractory /early relapse [HR-4.7, (95% CI-1,22); p=0.05] had significant impact on 5 yr FFTF whereas disease status at transplant (CR vs. No CR) had significant impact on 5 yr OS [HR-4.6, (95% CI-1.03, 20.5); p=0.04].
Conclusions:
Identification of predictors of poor response to salvage chemotherapy is an unmet need in the management of pediatric HL since complete response (CR) before transplant is independent predictor of survival. Stage 4 and bulky disease at relapse are high risk factors to predict incomplete response. Future trials should explore newer agents for effective salvage for these patients to attain complete response before ASCT.
Keywords
relapsed hodgkin lymphoma
risk factor
poor response.
Introduction
Pediatric Hodgkin lymphoma (HL) is one of the childhood malignancies with excellent cure rates with multiagent chemotherapy and radiotherapy. However, small subset of 5-10% of patients relapse after initial treatment (1). Treatment of these patients includes salvage chemotherapy followed by high dose chemotherapy and autologous stem cell transplant (ASCT) based on stage at relapse, time of relapse (early vs. late relapse) after initial diagnosis and response to salvage chemotherapy (1). There is wide heterogeneity in the salvage chemotherapy regimen used in relapsed/refractory pediatric HL by various pediatric oncology groups (2-6).
Most of the studies in relapse/refractory HL have reported outcomes of high dose chemotherapy and autologous stem cell transplant which are retrospective in nature (1,3, 5-13). Response to salvage chemotherapy is considered an important prognostic factor for long term outcome (14). Other factors which were prognostic for survival were early relapse (< 12 months from completion of treatment), elevated lactate dehydrogenase, presence of B symptoms, extranodal involvement and stage at relapse (1,5, 10, 15).
There is limited data regarding patients who received salvage chemotherapy but did not undergo ASCT due to progressive disease and hence overestimating the outcome of relapsed refractory HL. These are the patients who are likely to receive newer drugs like brentuximab, vedotin, nivolumab or participate in clinical drug trials. Identification of these patients prior to salvage chemotherapy may enable them to get initiated on newer regimens or drugs to elicit a response and further proceed to transplant, rather than initiating the same after failure of salvage regimens. Data regarding the predictors of poor response to salvage chemotherapy is limited and unmet need in relapsed HL.
We conducted a retrospective study to identify prognostic factors predicting response to salvage chemotherapy as well as survival in relapsed/refractory pediatric HL.
Methods
This retrospective study was performed in a tertiary cancer centre and was approved by Institutional Ethics Committee. We included patients with tissue diagnosis of HL, age ≤18 years at diagnosis who relapsed or had progressive disease after first line of chemotherapy between January, 2003 and December, 2013.
Data Collection
Medical records of all patients diagnosed with HL were retrieved. Data regarding the presenting symptoms, baseline laboratory parameters [complete blood count, lactate dehydrogenase (LDH), erythrocyte sedimentation rate (ESR), and serum albumin], histological subtype, and date of start and completion of chemotherapy were recorded. Staging was done using modified Ann Arbor classification (16).
Definition of Primary Refractory and Relapsed Hodgkin Lymphoma
Patients who progressed during first line therapy or who progressed after initial response within 3 months of completion of therapy were defined as having primary refractory disease. Progressive disease was defined as appearance of any new lesion or increase in size of pre-existing disease site on CECT or PET-CT imaging. Patients who experienced relapse from 3 months to 12 months after completion of therapy were defined as early relapse and those who relapsed after 12 months were defined as late relapse.
Evaluation and Treatment of Relapse
All patients who relapsed had complete re-staging with contrast enhanced computed tomography of neck, chest, abdomen and pelvis along with bone marrow aspirate and biopsy. Modified Ann Arbor classification was used for re-staging (16). Patients with primary refractory disease and advanced stage (IIB-IV) at relapse irrespective of timing of relapse (early vs. late) underwent salvage chemotherapy and based on response underwent ASCT. Patients with late relapse and early stage (stage IA, IB and IIA) at time of relapse received only salvage chemotherapy. These patients were excluded from analysis as they were not intended for consolidation with ASCT.
Response assessment with CECT or PET- CT was done after 2-3 cycles of salvage chemotherapy. Response was defined on CECT or PET-CT as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). CR was defined as resolution of previous involved disease site on CECT or absence of any metabolic activity or uptake less than uptake in mediastinal blood pool on PET-CT, partial response was defined as >50% decrease in size of measurable disease on CECT or decrease in size and uptake on PET-CT imaging, progressive disease was defined as appearance of any new lesion or increase in size of pre-existing disease site on CECT or PET-CT imaging (17).
Statistical Analysis
Freedom from treatment failure (FFTF) for the entire was defined as time from date of relapse to disease progression, relapse or death from any cause. For FFTF post transplant (patients who underwent transplant were included) was defined as time from date of transplant to disease progression, relapse or death from any cause. Overall survival (OS) was defined as time from date of relapse to date of last follow-up or death from any cause. Data was censored on 31st December, 2016. Logistic regression analysis was used to identify factors predicting response to salvage chemotherapy. Cox proportional hazards model was used for univariate analysis to identify predictive factors for FFTF and OS. Factors with significance with p value <0.05 were considered for multivariate Cox regression analysis to identify predictive factors for FFTF and OS. Stata (v 11.2, Statacorp, Chicago, IL, USA) was used for data analyses.
Results
A total of 50 patients had relapse of HL. Four patients had late relapse and stage 2A at the time of relapse; they received salvage chemotherapy, attained CR and they were not candidates for ASCT as they were at early stage at the time of late relapse. These patients were excluded from the analysis. Of the remaining 46 patients, 13 (28.3%) had primary refractory disease, 14 (30.4%) had early relapse and 19 (41.3%) had late relapse. Baseline characteristics of patients at diagnosis are given in Table 1. Disease and treatment characteristics at the time of relapse are given in Table 2.
| Baseline characteristic (N=46) | Median, N (%) |
|---|---|
| Median Age (yr) (range) | 12 (4-18) |
| Sex • Male • Female |
36 (80.7) 10(19.3) |
| B symptoms | 37 (71.1) |
| Stage • I • II • III • IV |
2(4) 15(30) 24(48) 9(18) |
| Bulky disease | 15 (30) |
| Extranodal disease (excluding Liver, Bone marrow) | 10(19.3) |
| Bone marrow involvement | 2(4) |
| Liver involvement | 4 (3.85) |
| Spleen involvement | 23 (44.2) |
| Subtype • Nodular sclerosis • Mixed cellularity • Lymphocyte rich • Lymphocyte depleted • NLPHL • Unspecified |
10(19.3) 28(56.4) - 1(2) - 11(22.3) |
| Involved field radiotherapy | 12 (23) |
| ABVD like regimen | 40 (76.9%) |
| Characteristic(N=46) | Median, N (%) |
|---|---|
| Timing of relapse • Primary refractory disease • Early relapse • Late relapse |
13(28.3) 14(30.4) 19(41.6) |
| Stage • I • II • III • IV |
1(4.4) 12(26.7) 17(37.8) 14(31.1) |
| Extranodal disease | 11(21.1) |
| Bone marrow involvement | 4(7.7) |
| Liver involvement | 7(13.4) |
| Spleen involvement | 21(46.7) |
| Salvage chemotherapy • Platinum based chemotherapy • ABVD • Other |
27(60) 14(31.2) 4(8.8) |
A total of 45 patients received salvage chemotherapy. Twenty seven patients (58.6%) received platinum based salvage chemotherapy while 14 received ABVD (30.4%) as shown in Table 2. One patient did not receive any salvage chemotherapy and died of progressive disease. Median follow up of cohort was 41 months (range-4-141 months).
Response to Salvage Chemotherapy
Overall response rate to salvage chemotherapy was 59.9 % with CR in 20 (44.4%) patients and PR in 7 (15.5%) patients. Twenty-one patients received second salvage chemotherapy and of them 4 attained CR (19%) and 5 attained PR (23.8%). Thirty four (76.3%) patients received ASCT (CR-21, PR-11, and PD- 2).
Factors predicting complete response to chemotherapy
On logistic regression analysis, stage 4 disease at relapse (p=0.02) and bulky disease at relapse (p=0.03) were predictors for not attaining CR to salvage chemotherapy (Table 3).
| Parameter | CR*, n (%) | No CR, n (%) | OR(95% CI) | P value |
|---|---|---|---|---|
|
Gender • Male(n=36) • Female(n=9) |
19(52.8) 2(22.2) |
17(47.2) 7(77.8) |
3.9 (0.8-24) |
0.1 |
|
B symptoms • Present(n=32) • Absent(n=13) |
14(43.7) 7(53.8) |
18(56.2) 6(46.2) |
1.5 (0.4-5.7) |
0.5 |
|
Bulky disease at baseline • Present(n=12) • Absent(n=33) |
7(58.3) 14(42.4) |
5(41.7) 19(57.6) |
0.6 (0.2-2.2) |
0.5 |
|
Extranodal disease at baseline • Present(n=9) • Absent(n=36) |
2(22.2) 19(52.8) |
7 (77.8) 17(47.2) |
3.9 (0.7-20.1) |
0.1 |
|
Spleen involvement at baseline • Present(n=21) • Absent(n=24) |
13(61.9) 8(33.3) |
8 (38.1) 19(66.7) |
0.3 (0.1-1.04) |
0.06 |
|
Stage 4 disease at baseline • Present(n=7) • Absent(n=37) |
2(28.6) 19(51.3) |
5(71.4) 18(48.7) |
2.6 (0.5-17.7) |
0.2 |
|
Timing of relapse • Primary refractory/Early relapse(n=26) • Late relapse (n=19) |
10(38.4) 11(57.9) | 16(61.6) 8 (42 .1) | 2.2 (0.7-7.7) |
0.2 |
|
Extranodal disease at relapse • Present(n=10) • Absent(n=35) |
3(30) 18(51.4) |
7 (70) 17(48.6) |
2.4 (0.5-10.4) |
0.2 |
|
Spleen involvement at relapse • Present(n=18) • Absent(n=27) |
8(44.4) 13(48.2) |
10 (55.6) 14(51.8) |
1.1 (0.3-3.5) |
0.9 |
|
Stage 4 disease at relapse • Present(n=15) • Absent(n=30) |
4(26.7) 17(56.7) |
11(73.3) 13(43.3) |
5.1 (1.3-23.9) |
0.02 |
|
Bulky disea se at relapse • Present(n=10) • Absent(n=35) |
2(20) 19(54.3) |
8(80) 16(45.7) |
7.1 (1.2-42) |
0.03 |
CR- complete response
Freedom from treatment failure and overall survival
FFTF at 5 yr for the entire cohort was 49.8% and OS at 5 yr was 66.3%. FFTF post transplant and OS at 5 yr for patients who underwent ASCT were 65.3% and 80.7%, respectively.
Prognostic factors for survival for entire cohort
On univariate analysis, female sex, extranodal disease at baseline, stage 4 disease at relapse and chemorefractory disease had inferior FFTF (Table 4). On multivariate analysis, stage 4 disease at relapse (p=0.047) and response to salvage chemotherapy (p<0.0001) had significant impact on FFTF (Table 5, Fig. 1). For OS only response to salvage chemotherapy (p<0.0001) had significant impact on OS (Table 4).
| FFTF at 5 yr | OS at 5 yr | |||||
|---|---|---|---|---|---|---|
| Estimate | HR (95% CI) | P value | Estimate | HR (95% CI) | P value | |
|
Gender • Male (N=36) • Female (N=9) |
54.9±0.08 30.1±0.14 |
2.3 (1.0-5.5) |
0.04 | 65.6±0.08 66.7±0.19 |
2.3 (0.85-6.3) |
0.09 |
|
B symptoms • Present(n=32) • Absent(n=13) |
61.5±0.13 43.6±0.08 |
1.1 (0.4-2.6) |
0.8 | 69.2±0.12 63.1±0.08 |
0.9 (0.3-2.4) |
0.8 |
|
Extranodal disease at baseline • Present(n=9) • Absent(n=36) |
16.6±0.13 57.3±0.08 |
2.5 (1-5.6) |
0.04 | 83.3±0.15 61.1±0.08 |
0.6 (0.1-2.4) |
0.4 |
|
Spleen involvement at baseline • Present(n=21) • Absent(n=24) |
48.4±0.13 49.2±0.12 |
0.9 (0.4-2.6) |
0.8 | 60.7±0.15 68.0±0.12 |
1.1 (0.4-2.8) |
0.7 |
|
Bulky disease at baseline • Present(n=12) • Absent(n=33) |
53.8±0.13 47.6±0.08 |
1.2 (0.5-2.9) |
0.6 | 58.7±0.14 67.2±0.08 |
1.8 (0.7-5.0) |
0.2 |
|
Timing of relapse • Primary refractory/Early relapse(n=26) • Late relapse (n=19) |
36.2±0.09 68.4±0.10 |
2.1 (0.9-5.6) |
0.07 | 62.2±0.10 68.4±0.10 |
1.2 (0.5-3.3) |
0.6 |
|
Bulky disease at relapse • Present(n=10) • Absent(n=35) |
50.0±0.15 48.8±0.08 |
0.9 (0.3-2 .4) |
0.8 | 53.3±0.17 68.2±0.08 |
1.2 (0.4-3.6) |
0.8 |
|
Spleen in volvement at relapse • Present(n=18) • Absent(n=27) |
43.7±0.09 52.5±0.11 |
1.0 (0.4-2.4) |
0.8 | 53.8±0.17 68.2±0.08 |
1.4 (0.5-3.5) |
0.4 |
|
Stage at relapse • Stage 2 and 3 (n=30) • Stage 4 (n=15) |
63.1±0.09 23.4±0.11 |
3.3 (1.5-7.6) |
0.004 | 72.1±0.08 49.3±0.13 |
2.8 (1.04-7.6) |
0.04 |
|
Response to chemotherapy* • Complete response (n=21) • Partial response (n=11) • Progressive disease (n=13) |
78.5±0. 09 36.3±0.14 7.1±0.09 |
- 4.9(1.4-17.1) 17.8(5.6-56.4) |
- 0.01 <0.0001 |
90.5±0.06 58.5±0.16 8.7±0.08 |
- 3.8(0.8-17.5) 11.8(4.9-65.5) |
- 0.08 <0.0001 |
*One patient who did not receive salvage chemotherapy was excluded from analysis
| FFTF at 5 yr | |||
|---|---|---|---|
| HR | 95% CI | P value | |
|
Sex • Male (N=36) • Female (N=9) |
1 1.74 |
- 0.6,4.6 |
0.25 |
|
Stage at relapse • Stage 2 and 3 (n=30) • Stage 4 (n=15) |
1 2.4 |
- 1.1,6.9 |
0.047 |
|
Response to chemotherapy • Complete response (n=21) • Partial response (n=11) • Progressive disease (n=13) |
1 4.3 16.6 |
- 1.2,15 5.1,53.8 |
- 0.02 <0.0001 |
|
Extranodal disease at baseline • Absent(n=36) • Present(n=9) |
1 2.4 |
- 0.9,6.8 |
0.9 |
Prognostic factors for survival for patients who underwent transplant
Among patients who underwent transplant, patients receiving more than 1 salvage chemotherapy to attain any response (CR and PR) [HR-3.8, (95% CI-1.2, 12.4); p=0.02], disease status at transplant (CR vs. No CR) [HR-3.4, (95% CI- 1.1, 11.1); p=0.04] and those who had early relapse/primary refractory disease [HR-5.4 (95% CI-1.2-24.8); p=0.03] had significant impact on 5 yr FFTF post transplant in univariate analysis, whereas only disease status at transplant (CR vs. No CR) alone had significant impact on 5 yr OS [HR-4.6, (95% CI- 1.03, 20.5); p=0.04] (Supplementary Table 1). On multivariate analysis only patients with primary refractory disease/early relapse had inferior 5 yr FFTF post transplant as compared to late relapse [HR-4.7 (95% CI-1, 22); p=0.05] (Supplementary Table 2).
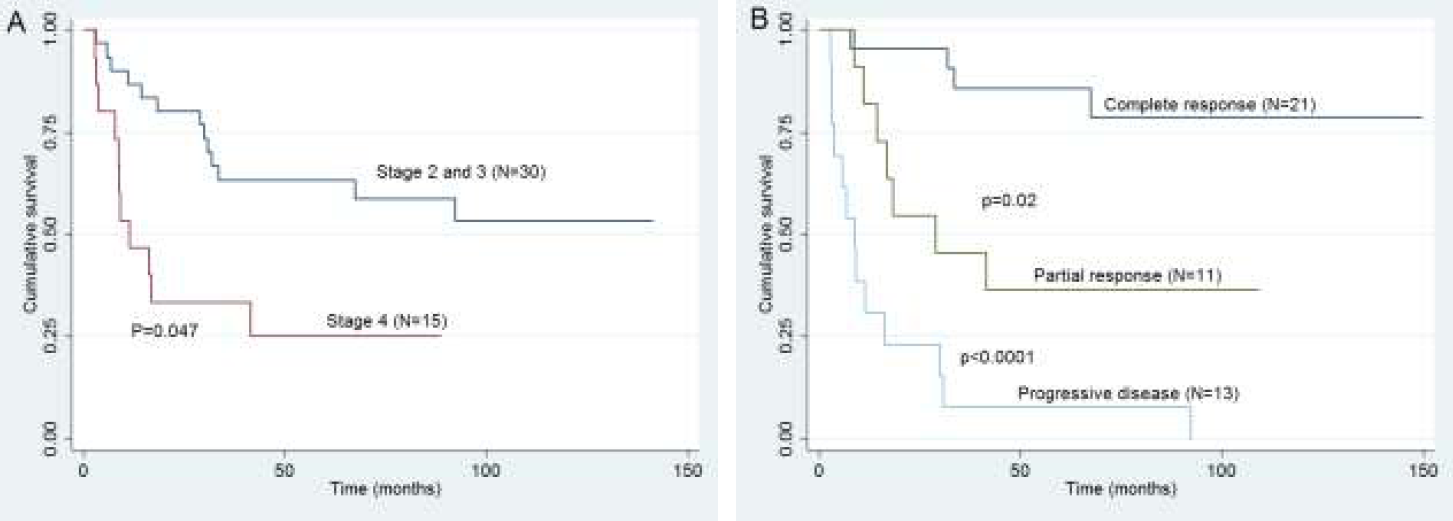
- Kaplan Meier survival graphs for 5 yr FFTF based on (A) Stage 2 and 3 vs. stage 4 at relapse (B) Response to salvage chemotherapy (complete response vs. partial response vs. progressive disease).
| FFTF at 5 yr | OS at 5 yr | |||||
|---|---|---|---|---|---|---|
| Estimate | HR (95% CI) | P value | Estimate | HR (95% CI) | P value | |
|
Gender • Male (N=28) • Female (N=6) |
66.8±0.09 62.5±0.21 |
1.5 (0.4-5.8) |
0.68 | 75.9±0.08 66.6±0.27 |
1.4 (0.3-7.1) |
0.42 |
|
B symptoms • Present(n=25) • Absent(n=9) |
60.6±0.10 77.8±0.13 |
1.2 (0.3-4.2) |
0.8 | 77.5±0.08 88.8±0.10 |
1.2 (0.2-5.7) |
0.8 |
|
Extranodal disease at baseline • Present(n=6) • Absent(n=28) |
66.6±0.13 70.9±0.08 |
2.5 (1-5.6) |
0.2 | 50±0.35 76.9±0.08 |
0.6 (0.1-2.4) |
0.6 |
|
Spleen involvement at baseline • Present(n=18) • Absent(n=16) |
58.3±0.12 72.2±0.12 |
1.8 (0.5-6.1) |
0.3 | 74.5±0.13 86.5±0.08 |
1.8 (0.4-7.4) |
0.4 |
|
Bulky disease at baseline • Present(n=9) • Absent(n=25) |
61.7±0.10 51.8±0.08 |
1 (0.3-3.7) |
0.9 | 77.8±0.13 81.9±0.08 |
1.9 (0.5-8.3) |
0.3 |
|
Timing of relapse • Primary refractory/Early relapse(n=20) • Late relapse (n=14) |
44.3±0.12 79.6±0.13 |
5.5 (1.2-24.8) |
0.03 | 71.2±0.10 92.4±0.10 |
1.9 (0.5-8.3) |
0.2 |
|
Bulky disease at relapse • Present(n=7) • Absent(n=27) |
71.4±0.17 63.3±0.09 |
0.7 (0.2-3.3) |
0.6 | 68.5±0.18 84.4±0.07 |
1.2 (0.2-6.1) |
0.8 |
|
Spleen involvement at relapse • Present(n=14) • Absent(n=20) |
41.6±0.2 65.3±0.1 |
2.3 (0.7-8.1) |
0.2 | 69.2±0.12 88.6±0.07 |
2.0 (0.5-7.6) |
0.3 |
|
Stage at rel apse • Stage 2 and 3 (n=8) • Stage 4 (n=26) |
41.6±0.20 65.8±0.10 |
2.3 (0.7-8.1) |
0.17 | 87.5±0.11 79.1±0.08 |
1.6 (0.3-8.4) |
0.5 |
|
Disease status before transplant • Complete response (n=22) • No complete response (n=12) |
80.8±0.08 38.1±0.09 |
3.5 (1.1-11.4) |
0.03 | 90.1±0.06 63.5±0.14 |
4.6 (1.03-20.5) |
0.04 |
|
No of lines of salvage therapy • 1(n=24) • >1 (n=10) |
71.6±0.10 22.5±0.18 |
4.0 (1.2-12.9) |
0.02 | 82.4±0.08 77.1±0.14 |
1.9 (0.5-7.9) |
0.3 |
| FFTF at 5 yr | |||
|---|---|---|---|
| HR | 95% CI | P value | |
|
Timing of relapse • Late relapse (n=14) • Primary refractory/Early relapse(n=20) |
1 4.4 |
- 0.9,22.0 |
0.06 |
|
Disease status before transplant • Complete response (n=22) • No complete response (n=12) |
1 3.0 |
- 1.3,9.7 |
0.07 |
|
No. of lines of salvage therapy • 1 (n=24) • >1 (n=10) |
1 1.8 |
- 0.5,6.9 |
0.4 |
Discussion
With the availability of salvage chemotherapy followed by high dose chemotherapy with stem cell rescue, significant proportion of relapsed HL patients can be cured. However, not all the patients reach up to the phase of consolidation with ASCT. Previous studies reported the outcomes of only patients who underwent ASCT (1, 3, 7-11). Our study included all the patients with relapsed/refractory HL for outcome analysis.
OS and FFTF of the entire cohort is less than that reported in previous studies (1,5-10,13,14). However, in our study, patients who underwent transplant had similar survival as compared to previously reported studies (1, 3, 5, 10, 18). Inclusion of all patients with relapsed/refractory HL gives more realistic outcome.
Complete response prior to ASCT is an important prognostic factor for survival as demonstrated in previous studies (1, 10, 14). Identification of predictors of poor response to salvage chemotherapy is an unmet need in the management of relapsed pediatric HL. We identified stage 4 and bulky disease at relapse as independent predictors of achieving complete response to salvage chemotherapy. Our results show that a significant proportion of patients (26.1%) do not reach upto the stage of consolidation with ASCT. These are the patients who did not respond despite receiving multiple lines of salvage chemotherapy. Notably, the subsequent complete response rate in those who fail the first salvage regimen was only 20% in our cohort. Thus, relapse patients with these high risk features are the ones who are candidates for exploring newer agents such as Brentuximab vedotin and Bendamustine so as to attain complete response before consolidation with ASCT.
Stage 4 disease at relapse and chemorefractory disease were independent predictors of inferior FFTF and OS in our cohort. Timing of relapse (early vs. late) was not a significant prognostic factor in our study as seen in previous studies. Notably in our study 50% patients of stage 4 disease at relapse did not reach up to consolidation with ASCT. Since stage 4
disease also has poor response to salvage chemotherapy, this subgroup of patients requires newer agents as salvage to attain complete response.
Disease status at time of transplant was independent predictor of OS. This further suggests that to attain complete response with first salvage chemotherapy is an important milestone to have better long term survival.
Ours is the first study to evaluate risk factors to predict complete response to salvage chemotherapy in pediatric HL, and has analyzed outcome of relapsed HL in a real life situation by including all patients with relapse/refractory disease, and not just those who reach upto the stage of transplant. To conclude, complete response to salvage chemotherapy is an important predictor of long term survival in relapsed pediatric HL, and with salvage chemotherapy majority of patients reach upto the stage of transplant. Stage 4 and bulky disease at relapse are high risk factors to predict incomplete response. Effective salvage chemotherapy regimen to attain complete response before ASCT is an unmet need in these patients. Future trials exploring newer agents such as Brentuximab and Bendamustine should be explored specifically as salvage options in these high risk patients prior to subjecting them to other conventional chemotherapeutic options.
References
- A prognostic model predicting autologous transplantation outcomes in children, adolescents and young adults with Hodgkin lymphoma. Bone Marrow Transplant. 2015;50(11):1416-1423.
- [Google Scholar]
- APE chemotherapy for children with relapsed Hodgkin disease: a Pediatric Oncology Group trial. Pediatr Blood Cancer. 2006;46(3):320-324.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome after autologous hemopoietic stem cell transplantation in relapsed or refractory childhood Hodgkin disease. J Pediatr Hematol Oncol. 2004;26(11):740-745.
- [CrossRef] [PubMed] [Google Scholar]
- Ifosfamide and vinorelbine is an effective reinduction regimen in children with refractory/relapsed Hodgkin lymphoma, AHOD00P1: a children's oncology group report. Pediatr Blood Cancer. 2015;62(1):60-64.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of children and adolescents with recurrent/refractory classical Hodgkin lymphoma, a study from the Société FranÇaise de Lutte contre le Cancer des Enfants et des Adolescents (SFCE) Br J Haematol. 2012;158(5):649-656.
- [CrossRef] [PubMed] [Google Scholar]
- Salvage therapy of progressive and recurrent Hodgkin's disease: results from a multicenter study of the pediatric DAL/GPOH-HD study group. J Clin Oncol. 2005;23(25):6181-6189.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous bone marrow transplantation for pediatric Hodgkin's disease: a case-matched comparison with adult patients by the European Bone Marrow Transplant Group Lymphoma Registry. J Clin Oncol. 1993;11(11):2243-2249.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous hematopoietic stem-cell transplantation for relapsed or refractory Hodgkin's disease in children and adolescents. J Clin Oncol. 1999;17(3):825-831.
- [CrossRef] [PubMed] [Google Scholar]
- High-dose chemotherapy and autologous stem cell transplant in adolescent patients with relapsed or refractory Hodgkin's lymphoma. Bone Marrow Transplant. 2010;45(3):476-482.
- [CrossRef] [PubMed] [Google Scholar]
- High-dose therapy and autologous hematopoietic stem-cell transplantation for recurrent or refractory pediatric Hodgkin's disease: results and prognostic indices. J Clin Oncol. 2004;22(22):4532-4540.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12(10):1065-1072.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of autologous hematopoietic stem cell transplantation in children and adolescents with relapsed or refractory Hodgkin's lymphoma. Pediatr Transplant. 2015;19(7):745-752.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of hematopoietic stem cell transplant as salvage therapy for Hodgkin's lymphoma in adolescents and young adults at a single institution. Leuk Lymphoma. 2010;51(4):664-670.
- [CrossRef] [PubMed] [Google Scholar]
- Initial response to salvage therapy determines prognosis in relapsed pediatric Hodgkin lymphoma patients. Cancer. 2010;116(18):4376-4384.
- [CrossRef] [PubMed] [Google Scholar]
- Stratification of treatment intensity in relapsed pediatric Hodgkin lymphoma. Pediatr Blood Cancer. 2014;61(4):579-586.
- [CrossRef] [PubMed] [Google Scholar]
- Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989;7(11):1630-1636.
- [CrossRef] [PubMed] [Google Scholar]
- Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of autologous hematopoietic stem cell transplantation in children and adolescents with relapsed or refractory Hodgkin's lymphoma. Pediatr Transplant. 2015;19(7):745-752.
- [Google Scholar]





