Translate this page into:
Prevalence of various fungal infections among HIV/AIDS patients
* Corresponding author: Dr. Shalini Malhotra, Department of Microbiology, Atal Bihari Vajpayee Institute of Medical Sciences and Dr. Ram Manohar Lohia Hospital, Delhi, India. drshalinimalhotra@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Kumari N, Malhotra S, Chauhan AK, Chauhan N, Bhatia NK, Prevalence of various fungal infections among HIV/AIDS patients. Ann Natl Acad Med Sci (India). 2025;61:106-12. doi: 10.25259/ANAMS-2023-5-11-(931)
Abstract
Objectives
Human immunodeficiency virus (HIV)-associated opportunistic fungal infections (OFI) are a major cause of mortality and morbidity in HIV-seropositive patients. This prospective study aimed to isolate various fungal pathogens from HIV seropositive patients and to identify and characterize these fungal pathogens at the species level in India.
Material and Methods
Based on clinical signs and symptoms, various clinical specimens (n=323) were collected from (n=200) HIV-seropositive patients in the adult age group of either sex and underwent direct microscopy and fungal culture. Fungal isolates were identified and specified according to a standard protocol. Statistical analysis: All data were collected and analyzed using Microsoft Excel.
Results
Out of 323 samples from 200 HIV-seropositive patients with a suspected fungal infection, fungal isolates were found in 89 cases, or 27.56% of cases. The most frequently isolated fungal organism was Candida species (75.28%), followed by Cryptococcus neoformans (17.97%), Aspergillus species (4.48%), Alterneria species (1.12%), and Trichophyton mentagrophyte (1.12%). Amongst 67 Candida species, Candida albicans had the highest isolation rate (88.05%), followed by Candida tropicalis (5.97%), Candida parasilosis (2.98%), and Candida auris (2.98%).
Conclusion
Oropharyngeal candidiasis followed by cryptococcal meningitis was the most common OFI among other fungal infections. This study would help clinicians in the proper diagnosis and early treatment of these infections to prevent their devastating effects in developing countries like India.
Keywords
Aspergillus
Candida
Cryptococcus
Opportunistic infections
INTRODUCTION
Acquired immunodeficiency syndrome (AIDS) is an immune system disorder that affects humans, leading to a loss of CD4 cells below 200 cells/mm3 and a decrease in immunity.1,2
In 2016, one million people died from AIDS-related diseases globally.3 According to the United Nations Programme on HIV/AIDS (UNAIDS), an estimated 38 million people were living with human immunodeficiency virus (HIV) infection worldwide in 2019. It has been observed that opportunistic infections, particularly fungal infections, are the leading cause of morbidity and mortality in HIV-infected patients due to the progressive decline in CD4+ T cells.4
With an estimated 2.6 million HIV-infected individuals, India has the third-largest population of HIV-positive patients in the world.2 Opportunistic infections in people with late-stage HIV infection are primarily caused by fungi.3,4 A rapid decline in peripheral blood CD4+T cells is a key mechanism associated with the occurrence and progression of opportunistic fungal infections (OFI) in HIV seropositive patients.5-7 Opportunistic respiratory infections are major causes of morbidity and mortality in HIV patients, and pulmonary involvement is the first manifestation in approximately 65% of cases.8
The spectrum of fungal infections ranges from asymptomatic mucosal candidiasis to disseminated infections, fungal pneumonia, and meningitis.6 Fungal infections by Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus are the most prevalent mycoses in immunocompromised individuals.5 Systemic infections include Pneumocystis jirovecii (pneumocystosis), Cryptococcus neoformans (cryptococcosis), Histoplasma capsulatum (histoplasmosis), and Talaromyces, previously known as Penicillium marneffei (talaromycosis).1 The purpose of the present study was to characterize the various fungal pathogens found in HIV-positive patients and their susceptibility to different antifungal drugs.
MATERIAL AND METHODS
Study population and design
This cross-sectional observational study was carried out from November 01, 2019, to March 31, 2021, after obtaining approval and ethical clearance from the Institutional Ethics Committee (No. TP (MD/MS) 34/2019/IEC/ABVIMS/RMLH 698/19). According to various studies, the isolation rate of fungal infections in HIV-positive patients was 41.1%. Based on this figure, a minimum sample size of 166 samples was determined for analysis, allowing a 7.5% margin of error and a 5% significance level. The calculation was performed using the formula,
Where Zα = Value of Z at two-sided alpha error of 5%
Two hundred HIV seropositive patients in the adult age group (>18 years) of either sex, with suspected fungal infection, were enrolled in the study. The CD4 cell count was conducted at the Integrated counseling and testing center using the flow cytometry method. A total of 323 different clinical specimens were received from these 200 HIV seropositive patients who exhibited relevant clinical signs and symptoms while attending the antiretroviral therapy (ART) clinic or being admitted to the department of medicine.
Isolation and identification of fungal isolates
Oral and throat swabs were obtained by swabbing the oropharyngeal mucosa. cerebrospinal fluid (CSF), sputum, urine, and blood samples were collected according to the protocol and placed directly into sterile containers. Strict confidentiality was maintained throughout the study. A standard procedure was followed to process the samples.
Microscopy
Depending on the type of specimen and the suspected fungal infection, all samples were examined directly under a microscope using Gram stains, potassium hydroxide preparations/Wet preparations, and India ink preparations.
Fungal culture
Fungal cultures were grown on sabouraud dextrose agar (SDA) (with and without antibiotics) and blood agar as appropriate. Chloramphenicol (50 mg/L), cycloheximide (500 mg/L) (Hi-media), and gentamicin (40 mg/L) were added to prepare SDA with antibiotics. Samples were streaked in duplicate on slants, incubated at 25°C and 37°C, and examined for growth after 48 hours, then weekly for up to 6 weeks until being discarded as negative. Specimens inoculated onto blood agar were incubated for 24–48 hours.5 Cream-colored, smooth pasty colonies grown on SDA slant or blood agar were Gram-stained and processed further for the identification of yeast. Molds were identified based on morphological appearances and microscopic features under lactophenol cotton blue stain and Riddles Slide culture in accordance with standard procedures.9 Yeast isolates were identified and classified based on germ tube formation, Calcofluor White Staining, morphology on corn meal agar with Tween 80 (Hi-Media), Hi-Crome Candida agar (Hi-Media), and automated VITEK 2 compact system (BioMerieux) according to standard protocol.9,10
RESULTS
Among the 200 HIV-positive individuals, 144 (72%) were predominantly male. Most patients, 105 (52.50%), were between the ages of 30 and 45 years, with 59 (29.50%) under 30 years [Table 1].
| Age category | Number of patients | Male | Female | Percentage |
|---|---|---|---|---|
| <30 Years | 59 | 41 | 18 | 29.50 |
| 31-45 Years | 105 | 79 | 26 | 52.50 |
| ears | 28 | 18 | 10 | 14.0 |
| >60 Years | 8 | 6 | 2 | 4.0 |
| Total | 200 | 144 | 56 |
HIV: Human immunodeficiency virus
Among the 200 HIV-seropositive patients, weight loss (50.56%) was the most prevalent clinical symptom, followed by oral ulceration (46.06%), fever (37.07%), and cough (29.21%) [Figure 1].
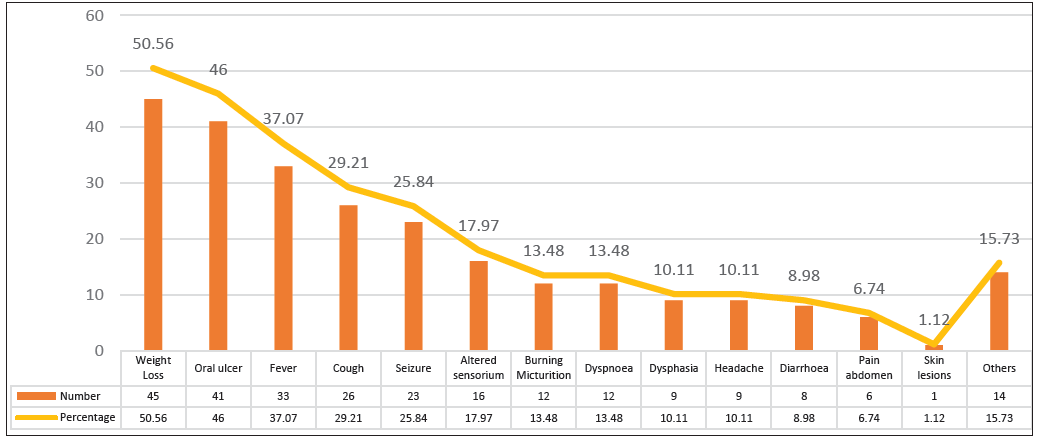
- Clinical profile of patients with confirmed fungal infections.
A total of 323 specimens were received from 200 HIV seropositive patients with suspected fungal infections. Among these, 89 specimens (27.56%) tested positive for various fungal infections.
The CD4 levels of the patients in this study ranged from 5 to 927 cells/mm3. The mean CD4 cell count among the 200 patients with suspected fungal infection was 221.20 cells/mm3. Specifically, 18% showed a count of less than 50, 35.5% had counts between 50 and 199, 36.5% had counts between 200 and 500, and 10% had a CD4 cell count above 500 cells/mm3. In the 89 confirmed fungal-positive patients, the mean CD4 cell count was 140.8, with 24.71% having a count of less than 50, 48.31% between 50 and 199, 23.59% between 200 and 500, and only 3.37% of having a count above 500 cells/mm3.
The most common specimens in the study were CSF (62), followed by oral swabs (59), urine (57), sputum (51), and blood (45). Oral swabs (66.10%) and sputum samples (45.10%) had the highest positivity rate for fungal isolates [Table 2].
| Sample type | No. of samples | No. of fungal isolates | Isolated fungal species |
|---|---|---|---|
| Oral swab | 59 | 39 |
C. albicans: 38 C. tropicalis: 01 |
| Sputum | 51 | 23 |
C. albicans: 38 C. parasilosis: 01 A. niger: 02 A. flavus: 01 A. fumigatus: 01 |
| CSF | 62 | 15 | C. neoformans: 15 |
| Urine | 57 | 7 |
C. albicans: 03 C. tropicalis: 02 C. parasilosis: 01 C. auris: 01 |
| Blood | 45 | 1 | C. neoformans: 01 |
| Throat swab | 02 | 1 | C. tropicalis: 01 |
| Skin scrapping | 02 | 1 | T. mentagrophyte: 01 |
| Axilla/Groin swab | 01 | 1 | C. auris: 01 |
| Nail | 01 | 1 | Alternaria spp:01 |
| Total | 280 | 89 |
CSF: Cerebrospinal fluid
Candida albicans was the most frequently isolated fungal species in oral swabs (97.43%) and sputum samples (78%). It was also seen that CSF samples (88%) were primarily positive for Cryptococcus neoformans.
Candida species were the most frequently isolated fungi, accounting for 75.28%, followed by Cryptococcus neoformans, which was identified in 16 patients (17.97%). Among the 67 Candida species isolated, Candida albicans had the highest isolation rate (88.05%), followed by Candida tropicalis (5.97%) [Figure 2].
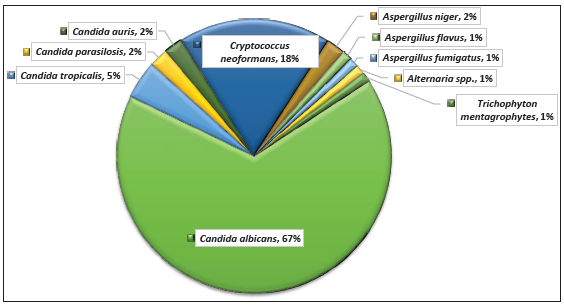
- Distribution of fungal species in human immunodeficiency virus (HIV) patients.
DISCUSSION
In developing countries, opportunistic fungal infections (OFI) in HIV-positive patients are the most important cause of mortality and morbidity.2-5 While there is extensive literature on the spectrum of OFIs (OFIs) in HIV seropositive patients worldwide, data from India remains inadequate.
In our study, 200 HIV-positive patients from different age groups were included, with a male predominance of 72%. Most patients (52.50%) were in the age group of 30-45 years, followed by 29.50% who were under 30 years. Similar results in the most sexually active and economically productive age male preponderance of 67% and 73.2% was observed by Kaur et al., (2016) and by Harikrishna et al., (2017) respectively.5,11 Our findings are consistent with other studies on HIV-seropositive patients in India and Iran.12,13 Men often migrate away from their homes in search of work, leading to prolonged separations from their wives and visits to brothels, which are significant contributors to HIV infection and the observed male preponderance. Additionally, many women in Indian society are housewives and may not receive treatment due to social stigma and lack of family support.14,15
Among the 200 HIV-seropositive patients with suspected fungal pathogens, the most prominent clinical features in our study were weight loss (50.56%) followed by oral ulcer (46.06%), fever (37.07%), and cough (29.21%). Similarly, a study from New Delhi, India, reported weight loss (78.2%) and oral ulcers (74.6%) as the prominent clinical features in HIV-positive patients.5 In a different study from Nepal, Joshi et al., (2004) also identified weight loss (58.8%) as the most common clinical feature, consistent with our findings.16 However, Chakravorty et al., (2006) reported that the most prevalent clinical feature was fever, observed in 70.6% of patients, followed by gradual weight loss in 53.3% of cases, while 18.0% of patients were asymptomatic.17
CSF samples (19.2 %) and oral swabs (18.26%) were the most frequently received samples in our study. The highest positive rates for fungal isolates were observed in oral swabs (66.10%), sputum (45.10%), and CSF (24.19%). In a study from New Delhi (2016), the most common sample was the oropharyngeal swab (100%), followed by induced sputum (32.5%), blood (31.8%), and CSF (26.2%). The highest rate of fungal isolation in that study was found in sputum samples (53.9%), followed by oropharyngeal swabs (49.3%), with only 9.6% of CSF samples testing positive for fungi.5 Among the 200 individuals suspected of having an opportunistic fungal infection, 89 patients had confirmed fungal infections. In our study, 71% of individuals with confirmed fungal infection had a CD4 count of less than 200 cells/mm3, indicating a correlation between low CD4 counts and OFIs.6,18 Most fungal isolates (i.e., Candida species) were obtained from oral swabs, which are commonly associated with oropharyngeal candidiasis, the most prevalent opportunistic fungal infection. According to several other studies, oropharyngeal candidiasis is the most common opportunistic fungal infection.1,18-22
Although Candida species are part of the normal flora in the oral cavity, we categorized them as opportunistic fungal pathogens because patients experienced oral ulcers with burning sensations during eating or swallowing. Oral candidiasis was found to be more prevalent in our study, likely due to patients having irregular access to ART center sessions during the COVID-19 era. Poor oral hygiene and a lack of awareness about opportunistic infections among HIV-seropositive patients may also contribute to the incidence of oral candidiasis. In our study, Candida albicans was the most frequently isolated fungal species from oral swabs (97.43%) and sputum samples (78%).
There were two isolates of Candida auris, one from urine, for which subsequent surveillance measures were initiated, and another isolated from an axilla/groin swab of the same patient, hence infection control measures for Candida auris were reinforced.
In our study, among the 62 CSF samples, 15 (24.19%) tested positive for fungal isolates, with the sole fungal species identified being Cryptococcus neoformans. However, several other studies have reported significantly lower isolation rates of 9.6%, 6.7%, 2.9%, 4%, and 3.7% for cryptococcal meningitis than the isolation rate observed in our study.5,18,19,23,24 The higher isolation rate observed in our study may be attributed to the study period coinciding with the COVID-19 era, during which most patients visited the hospital only when their condition worsened, experiencing seizures and altered sensorium that necessitated emergency medical monitoring. Given that cryptococcosis is the most common systemic fungal infection among AIDS patients, its prevalence is directly related to the rapid spread of the disease.18,25,26
In sputum samples, Candida species predominated at 82.6%, followed by Aspergillus species at 17.4%.
Upon further categorization, the percentage of Candida albicans was 78%, while non-albicans Candida accounted for 4.3%. Among the Aspergillus species, Aspergillus niger, Aspergillus fumigatus, and Aspergillus flavus were identified at 8.7 %,4.3%, and 4.3 %, respectively. These findings closely align with a study by Bharathi M. and Rani AU. (2011), which reported that 57% of isolates were Candida spp. Including 27% albicans and 30% non-albicans Candida, followed by 13.5% Aspergillus spp. as the major groups.27 In another study by Chandwani et al., (2016), Candida albicans was the most common isolate (31.7%), followed by Aspergillus niger (17.7%) and Aspergillus flavus (10%).6 In sputum samples, Candida species are typically commensals, while Aspergillus species are considered contaminants. However, both can act as opportunistic pathogens in immunocompromised individuals. In our study, the same fungal isolate was found in three consecutive sputum samples, and patients presented with symptoms of fever, cough, and chest pain, prompting us to report these isolates as fungal pathogens.
Candida species (75.28%), Cryptococcus neoformans (17.97%), Aspergillus species (4.48%), Alterneria species (1.12%), and Trichophyton mentagrophyte (1.12%) were the most frequently isolated fungal species in our study. These results are comparable to a previous study by Parmar et al., (2012), which reported Candida species (55%) as the most common fungal isolate, followed by Cryptococcus species (4%) and Aspergillus species (3%).2
Similarly, R. Kaur et al., (2016) from New Delhi found Candida species (86.5%) to be the most prevalent fungal isolate, followed by Aspergillus species (6.5%), Cryptococcus species (3.3%), Penicillium species (1.9%). Alterneria and Rhodutorula species were reported at 0.9% each5 [Table 3].
| Reference | Candida spp. | Cryptococcus spp. | Aspergillus spp. | Others |
|---|---|---|---|---|
| Our study | 75.28% | 17.97% | 4.49% | 2.24% |
| Kaur et al.5 (2016) | 86.5% | 3.3% | 6.5% | 3.7% |
| Gandham et al.31 (2013) | 71.7% | 1.2% | 14% | 13.1% |
| Parmar et al.2 (2012) | 83.3% | 6.0% | 4.7% | 6.0% |
| Bharathi and Usha27 (2011) | 57% | 5.2% | 13.5% | 24.3% |
| Jahromi and Khaksar28 (2005) | 69.4% | 4.2% | 13.9% | 12.5% |
HIV: Human immunodeficiency virus, AIDS: Acquired immunodeficiency syndrome
In another study from Iran by Jahromi SB and Khaksar AA (2005), Candida species accounted for 69.4%, followed by Aspergillus species (13.9%) and Cryptococcus species (4.2%).28 A study by Pagano et al., (2006) from Italy showed Aspergillus species as the most frequently isolated fungal species (57.6%), followed by Candida species (32.5%) and Cryptococcus species (1.4%).29 Additionally, a study by Kashyap et al., (2012) reported Candida species (18.3%) as the predominant fungal isolate, followed by Aspergillus species (6.9%) and Cryptococcus species (0.6%).30 The most isolated fungal species in the study by Gandham et al., (2013) was Candida species (71.7%), Aspergillus species (14%), Penicillium species (1.5%), Cryptococcus species (1.2%), and Rhodutorula species (0.9%).31
The isolation rate of Cryptococcus species in our study was 17.97%, which is significantly higher compared to the rates reported in other studies, which were 4%,3.3%,4.2%,1.4%,0.6%, and 1.2%.2,5,28-31 [Table 3].
Among the 67 Candida species isolated in our study, Candida albicans was predominant (88.05%), followed by Candida tropicalis (5.97%), Candida parasilosis (2.98%), and Candida auris (2.98%). Similarly, a study from New Delhi, India, found Candida albicans (75.8%) to be the most prevalent among Candida isolates, followed by Candida tropicalis (9.7%), Candida krusei (6.5%), Candida glabrata (4.3%), Candida parapsilosis (2.7%), and Candida kefyr (1.1%).5 Another study by Gandham et al., (2013) reported similar results, with Candida albicans at 51.4%.31 However, in contrast, Picardi et al., (2012) from the USA found non-albicans Candida to be the most prevalent isolate in neutropenic patients.32 Among Aspergillus species, Aspergillus niger was the most prominent at 50%, followed by Aspergillus flavus and Aspergillus fumigatus at 25% each. Another study from India also reported Aspergillus niger (50%) as the primary species, followed by Aspergillus fumigatus (35.7%) and Aspergillus flavus (14.3%).5 Meanwhile, Gandham et al., (2013) reported Aspergillus fumigatus (53.2%), followed by Aspergillus niger (25.5%) and Aspergillus flavus (14.9%).31 In our study, only Cryptococcus neoformans was isolated among Cryptococcus species(100%), which is comparable the findings of Gandham et al. (2013).31 However, Kaur et al., (2016), from New Delhi, India, reported both Cryptococcus neoformans (71.4%) and Cryptococcus gattii (28.6%) among Cryptococcus species (14.9%).5
In our study, one isolate of Alterneria species (1.12%) was obtained from a nail sample. In contrast, a study from New Delhi reported an isolation rate of 0.9% for Alternaria alternata from sputum samples. However, a study by Warthe N et al. (2015) found that 5.6% of cancer/HIV patients from central India had Alternaria alternata isolated from their blood.33 However, we did not find any case of Alternaria fungemia in HIV-seropositive patients.
CONCLUSION
Oropharyngeal candidiasis followed by cryptococcal meningitis are the most common opportunistic fungal infection in HIV-positive patients, particularly those with low CD4 counts. A detailed comparative study of CD4 cell counts, viral load, and OFIs could establish CD4 count as an indicator of opportunistic mycoses in HIV/AIDS patients. Such research would aid clinicians in t diagnosing and initiating early treatment for these infections, ultimately helping to prevent their severe consequences in developing countries like India.
Authors’ contributions
SM, NK: Concept and design; NK, SM, AKC, NC, NKB: Acquisition, analysis, or interpretation of data; NK, SM, AKC: Drafting of the manuscript; SM, NKB: Critical revision of the manuscript for important intellectual content; NC, AKC: Technical and material support; SM, NKB: Supervision.
Ethical approval
The research/study is approved by the Institutional Ethics Committee at Atal Bihari Vajpayee Institute of Medical Sciences and Dr Ram Manohar Lohia Hospital, number TP (MD/MS) 34/2019/IEC/ABVIMS/RMLH 698/19, dated 22nd October 2019.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- National AIDS Control Organisation, National Guidelines for HIV Testing NACO 2015, Available from: http//www.naco.gov.in/sites/default/files/National Guidelines for HIV Testing. [Last accessed on 2023 Nov 24].
- Prevalence of opportunistic fungal infection in HIV positive patient tertiary care hospital in Rajkot. Res Nat J Med. 2012;2:463-64.
- [Google Scholar]
- Human immunodeficiency virus disease-AIDS related disorders. In: Shamson JF, David KJ, eds. Harisson internal medicine (20th ed). USA: McGraw Hills Education; 2018. p. :3655-737.
- [Google Scholar]
- HIV UG. AIDS statistics. Fact sheet; 2020. Available from: http//www.unaids.org/en/resources/fact sheet [Last accessed 2023 Nov 24].
- Spectrum of opportunistic fungal infections in HIV/AIDS patients in tertiary care hospital in India. Can J Infect Dis Med Microbiol. 2016;2016:2373424.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mycological profile of sputum of HIV positive patients with lower respiratory tract infection and its correlation with CD4+ T lymphocyte count. J Clin Diagn Res. 2016;10:DC28-DC31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Reduced binding and phagocytosis of pneumocystis carinii by alveolar macrophages from persons infected with HIV-1 correlates with mannose receptor downregulation. J Clin Invest. 1998;102:1332-44.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory infection complicating HIV infection. Curr Opin Infect Dis. 2008;21:184-90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Laboratory technique. Medically important fungi a guide to identification (6th ed). Washington DC: ASM Press; 2018. p. :359-425.
- Vitek 2 Compact online software user manual & Biomerieux Inc. Vitek 2 compact hardware user manual. Available from: http://www.yeec.com [Last accessed 2025 Nov 23]
- Epidemiological study of opportunistic infections in HIV sero positive patients in South India. J Medical Sci Clin Res. 2017;05:22397-401.
- [CrossRef] [Google Scholar]
- Distributions and antifungal susceptibility of Candida species from mucosal sites in HIV positive patients. Arch Iran Med. 2010;13:282-7.
- [PubMed] [Google Scholar]
- Opportunistic infections in relation to antiretroviral status among AIDS patients from south India. Indian J Med Microbiol. 2011;29:395-400.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation between CD4 counts of HIV patients and enteric protozoan in different seasons - an experience of a tertiary care hospital in Varanasi (India) BMC Gastroenterol. 2008;8:36.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical and microbiological profile of HIV/AIDS cases with diarrhea in North India. J Pathog. 2012;2012:971958.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinico-epidemiological profile of HIV/AIDS patients in western Nepal a study from teaching hospital. Indian J Prev Soc Med. 2004;35:69-76. Available from: https://www.researchgate.net/publication/293798961
- [Google Scholar]
- Study on clinico-epidemiological profile of HIV patients in eastern India. J Assoc Physicians India. 2006;54:854-7.
- [PubMed] [Google Scholar]
- Spectrum of opportunistic fungal infections in HIV-infected patients and their correlation with CD4+ counts in western India. J Med Microbiol Infect Dis. 2014;2:19-22.
- [Google Scholar]
- A study of opportunistic infection in HIV seropositive patients. Indian J Comm Med. 2007;32:208-9.
- [Google Scholar]
- Spectrum of clinical and laboratory characteristics of HIV infection in northern India. J Commun Dis. 1995;27:131-41.
- [PubMed] [Google Scholar]
- AIDS-related opportunistic mycoses seen in a tertiary care hospital in North India. J Med Microbiol. 2007;56:1101-1106.
- [CrossRef] [PubMed] [Google Scholar]
- Spectrum of chief complaints and opportunistic infections among HIV seropositive patients attending a community care center in Nalgonda district, Andhra Pradesh. Int J Health Sci Res. 2015;5:288-91.
- [Google Scholar]
- Current trends of opportunistic infections among HIV-seropositive patients from Eastern India. Jpn J Infect Dis. 2008;61:49-53.
- [CrossRef] [PubMed] [Google Scholar]
- Spectrum of clinical disease in a series of 135 hospitalised HIV-infected patients from north India. BMC Infect Dis. 2004;4:52.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antifungal susceptibility testing: Current role from the clinical laboratory perspective. Mediterr J Hematol Infect Dis. 2014;6:e2014030.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Progress in antifungal susceptibility testing of Candida spp. by use of clinical and laboratory standards institute broth microdilution methods, 2010 to 2012. J Clin Microbiol. 2012;50:2846-56.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pathogenic fungal isolates in sputum of HIV positive patients. J AIDS HIV Res. 2011;3:107-13.
- [Google Scholar]
- Deep-seated fungal infections in immunocompromised patients in iran. Iran J Allergy Asthma Immunol. 2005;4:27-32.
- [PubMed] [Google Scholar]
- The epidemiology of fungal infections in patients with hematologic malignancies: The SEIFEM-2004 study. Haematologica. 2006;91:1068-75.
- [PubMed] [Google Scholar]
- Fungal profile of clinical specimens from a tertiary care hospital. Asian Pacific J Trop Biomed. 2012;2:S401-5.
- [CrossRef] [Google Scholar]
- The spectrum and aetiology of mycotic infections from a tertiary care hospital from western part of India. J Clin Diagn Res. 2013;7:2157-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Early ultrasonographic finding of septic thrombophlebitis is the main indicator of central venous catheter removal to reduce infection-related mortality in neutropenic patients with bloodstream infection. Ann Oncol. 2012;23:2122-2128.
- [CrossRef] [PubMed] [Google Scholar]
- Spectrum of opportunistic fungal infections in cancer/HIV patients: emerging fungal pathogens from Jabalpur Madhya Pradesh central India. Scholars J Applied Med Sci. 2015;3:1385-90.
- [Google Scholar]






