Translate this page into:
Recurrent Hypokalemic Paresis–A Possibility of Liddle-Gitelman Overlap
Address for correspondence Shiva Narang, MBBS, MD General Medicine, Department of Medicine, University College of Medical Sciences and GTB Hospital, Delhi 110095, India (e-mail: shivanarang@gmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Hypokalemia can occur due to various causes that have diverse manifestations varying from mild asthenias to acute onset paraparesis to life-threatening cardiac arrhythmias. A proper algorithmic evaluation for hypokalemia can help us identify various correctable causes and can be life-saving. Here, we discuss a case of 35-year-old hypertensive female who had presented as a case of acute onset flaccid paraparesis. We have further discussed our approach in reaching her diagnosis and stressed upon importance of algorithmic approach in the evaluation of patients presenting with recurrent hypokalemia. It may help in unmasking a rarer cause.
Keywords
Gitelman syndrome
hypokalemia
Liddle syndrome
Introduction
Hypokalemic paresis is a type of metabolic channelopathy that manifests as acute onset flaccid paresis which if untreated can lead to involvement of respiratory muscles and cardiac arrhythmias. It can either be due to primary or secondary causes. Secondary causes include thyrotoxic periodic paralysis, renal tubular acidosis, Gitelman syndrome, Liddle syndrome, diarrhea, barium poisoning, and primary hyperaldosteronism.1 The clinical manifestations of primary hypokalemia are similar to that of secondary hypokalemia but severity and management vary and therefore it is imperative to identify the cause. Here, we will discuss a case of hypokalemic periodic paresis with the possibility of one of the rarer causes.
Case Report
A 35-year-old married female presented with 1-day history of sudden onset quadriparesis, predominantly involving proximal part of lower limb. It was nonprogressive, not associated with any sensory disturbance, no bowel or bladder involvement. The patient was on a pilgrimage, climbing down a flight of stairs when she noticed weakness in bilateral lower limbs. It was associated with generalized body weakness and decreased grip strength. There was no associated fever, no associated abnormal body movements, no history of trauma, and no girdle-like sensation. No history of excessive meal intake, alcohol intake, or fasting prior to the occurrence of the weakness was reported. The patient provided history of similar episodes, first one occurring 13 years back at the age of 22 years, 6 months after the delivery of her first child; subsequent episode occurred 3 and 1 years back, respectively. Each event was associated with a hospital visit, where serum potassium levels were found to be low and the patient was supplemented with oral and intravenous potassium that relieved patient's symptoms; no subsequent recording of potassium levels post-correction was available. Patient was not on any supplementation or treatment in the intervening periods between the episodes. The patient was labeled as hypokalemic periodic paralysis in one of the hospital visits. No additional investigations were available with the patient. The patient was a known hypertensive and hypothyroid for the past 4 years on amlodipine 5 mg and euthyroid on 25 µg of levothyroxine. No other medications were being taken by the patient. No family history of similar weakness episodes in parents or siblings was present. On initial examination, the patient had a blood pressure of 171/100 mm Hg with pulse rate of 65/min and random blood sugar value of 201 mg/dL. She was conscious, oriented to time place person. Her power in the lower limb was 2/5 bilaterally and upper limb 3/5. There was areflexia and the tone was flaccid. There was no sensory deficit on examination. The patient's electrocardiography showed prominent U waves and her potassium levels recorded were 2.0 mEq/L. Magnesium levels were recorded to be 1.1 mEq/L, which were also low. Other electrolytes were within normal limits, and kidney function was normal. The patient was persistently in metabolic alkalosis. Further urinary studies of the patient were done, including urine spot potassium-creatinine ratio that was 57.5 mmol/g of creatinine (57.5 mEq/g of creatinine), indicating renal excretion of potassium, urinary sodium 76.1 mmol/L(76.1 mEq/L), and urine osmolality of 163 mOsm/kg H20. Transtubular potassium gradient calculated was 10.7 that confirmed renal loss of potassium. Urine calcium/creatinine ratio was found to be 0.17. Urinary chloride was increased to 99.8 mmol/L (99.8 mEq/L). Spot urine calcium and magnesium were 5 (2.5 mEq/L) and 4 mg/dL (3.2 mEq/L), respectively. Thyroid profile was suggestive of normal T3 T4 levels and thyroid-stimulating hormone of 5.64. Serum cortisol levels were found to be normal. Antinuclear antibodies of the patient were found to be negative using immunofluorescence assay. Further investigations revealed the patient to have grade 3 hypertensive retinopathy with Salu's and Gunn's sign positive. Ultrasound whole abdomen was suggestive of normal studies, and adrenal glands visualized to be normal.
During her hospital stay, she was initially supplemented with 160 mEq per day of potassium cumulatively through oral and central venous route. The symptoms of the patient improved gradually over a period of 3 days, but the potassium levels remained below 2.5 mEq/L, despite concomitant correction of serum magnesium. The potassium supplementation was further increased to 200 mEq/day with no subsequent improvement in the serum potassium levels. Her random blood glucose levels during stay were between 100 and 160 mg/dL with only one initial high recording and systolic blood pressure levels 140 to 149 mm Hg on amlodipine 10 mg daily. Patient was given spironolactone 50 mg once daily (od) for 3 days, but no improvement in the potassium levels was observed, despite daily supplementation. Spironolactone supplementation was stopped and the patient was started on amiloride 5 mg od with the continuation of potassium supplementation. Potassium levels were subsequently found to be in the range 3.5 to 4.5 mEq/L. Post initiation of the potassium-sparing diuretics, the 24-hour urinary potassium was found to be in the normal range. The patient was then discharged on amiloride 5 mg od and daily oral supplementation of 60 mEq of potassium. The patient was diagnosed as a case of salt-losing nephropathy with possibility of Liddle syndrome or Gitelman syndrome as overlapping features of both were found. Patient was followed 2 weeks post-discharge and had potassium levels of 3.8 mEq/L and blood pressure of 128/76 mm Hg. Patient is on regular follow-up and has had no episodes of hypokalemia after 4 months.
Discussion
Case report discusses a patient of hypokalemia paresis and her evaluation for the same. Our patient presented with flaccid areflexic paraparesis. On further evaluation, we found the cause for hypokalemia to be renal. She was further evaluated to look for exact cause of hypokalemia. We narrowed down to a possibility of Liddle syndrome in view of secondary hypertension and response to amiloride. The possibility of Gitelman was also considered in our case due to hypokalemia with normal calcium levels and hypomagnesemia.
Boorugu et al described a case of 53-year-old male with Liddle syndrome who presented with hypokalemic periodic paralysis.2 Liddle syndrome is characterized by hypertension, hypokalemia, and metabolic acidosis. Patient has mutations leading to constitutive activation of epithelial sodium channels that leads to sodium and water retention. This causes a low level of renin and angiotensin. Patients are usually diagnosed at younger ages as a cause of secondary hypertension. It rarely presents as hypokalemic paresis. Patients respond to potassium-sparing diuretics, similar to that seen in our patient. But to prove it to be Liddle, we need renin and angiotensin measurements. Kayal et al studied 56 cases of hypokalemic periodic paresis over a period of 24 months in a single-center prospective study.1 They tried to find out the causes of hypokalemia in the patients. About 42.9% of the reported cases had secondary causes of hypokalemia. Of this, 7.14% cases were due to Gitelman syndrome. It is characterized by metabolic alkalosis with hypokalemia, hypocalciuria, and hypomagnesemia. The reported incidence of Gitelman syndrome in Indian studies varies from 3.2 to 13.3%. Only two cases of Liddle syndrome presented as hypokalemic paralysis. This is a very rare presentation of Liddle as they usually present with muscle weakness due to hypokalemia rather than paralysis. They also reported that hypokalemia was more severe and required a longer duration of treatment to bring to normal levels in various secondary causes of hypokalemia.
Patra et al analyzed 200 patients presenting with hypokalemic periodic paralysis to determine the cause of hypokalemia.3 Gitelman syndrome was reported as the most important secondary cause of hypokalemia paralysis found in 28% of the studied population. The lack of genetic studies to determine Gitelman syndrome as the cause was a limitation, though no other cause could be determined as per the clinical parameters. Gitelman syndrome occurs to loss of function mutation of thiazide-specific sodium chloride channels in the distal convoluted tubules. Determining the cause of hypokalemia is important as it helps in guiding the treatment. Chandramohan et al in another study followed up patients of hypokalemic paresis to ascertain the cause in tertiary care center in India.4 They reported Gitelman syndrome as the second most common cause of hypokalemia in these patients. Another interesting finding reported by them was that patients with secondary causes of hypokalemia paresis had higher degree of renal potassium losses and took longer to treat.
In ►Figs. 1 and 2, we represent the algorithmic approach to evaluation of hypokalemia with simple laboratory tests.5
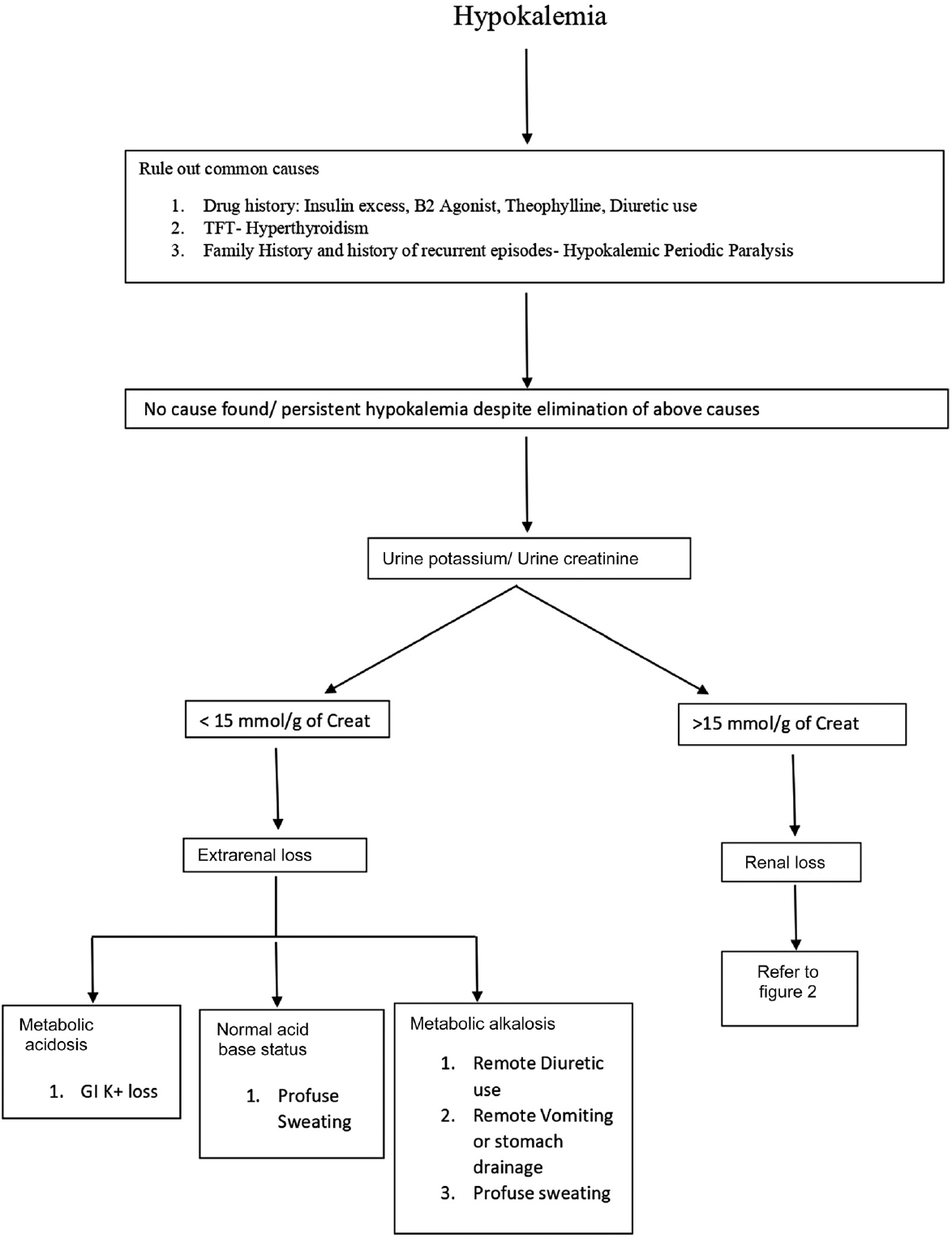
- Evaluation of hypokalemia. TFT, thyroid function test.
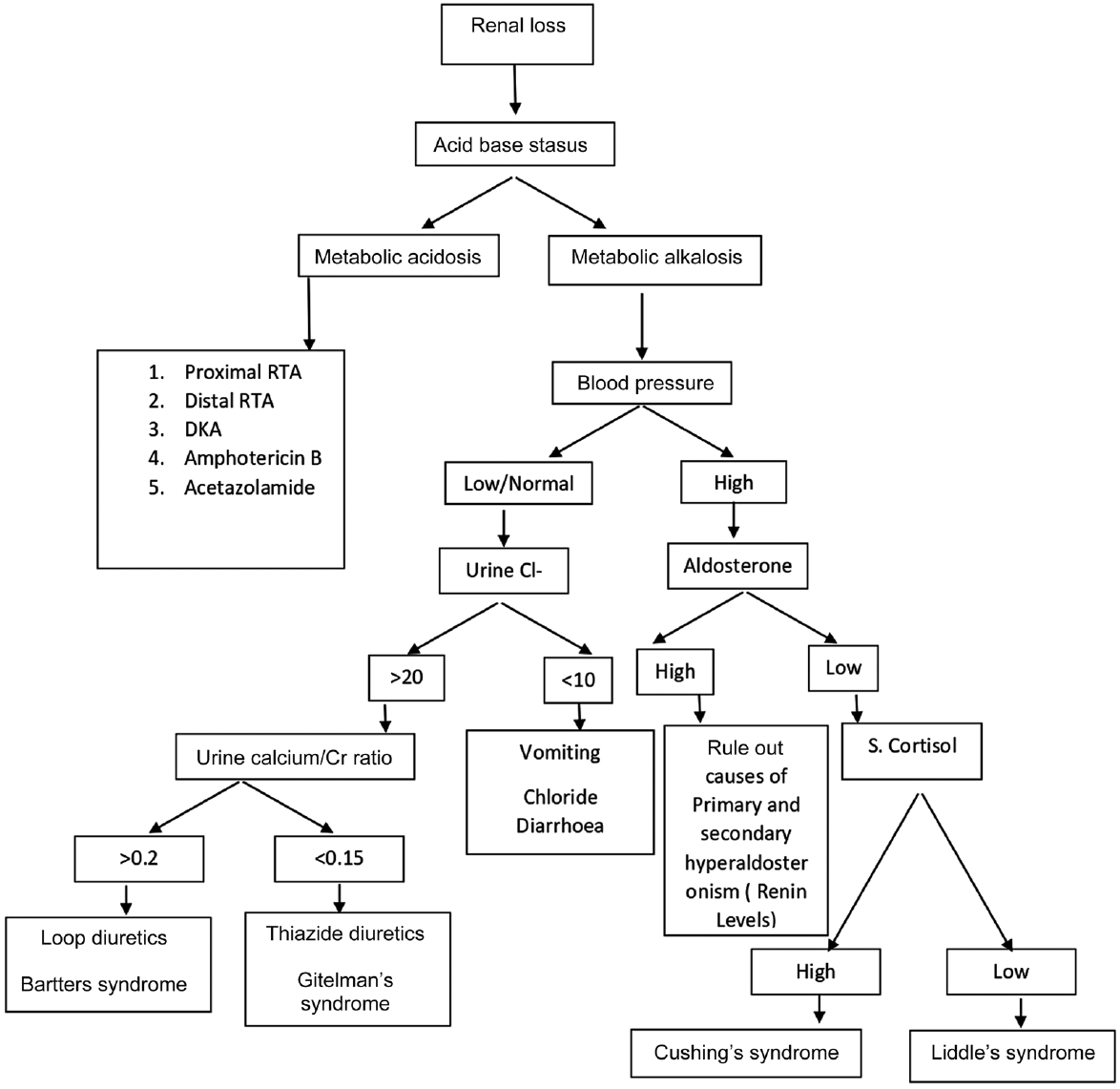
- Evaluation of renal causes of hypokalemia. DKA, Diabetic Ketoacidosis; RTA, renal tubular acidosis.
This case may be a rare case of hypokalemic paresis. Simple laboratory evaluation can lead to a fairly acceptable diagnostic possibility for the patient even if all tests are not feasible. Such detailed evaluation will help in long-term alleviation of the symptoms and prevention of further such episodes. Due to nonavailability of genetic profiling and renin angiotensin aldosterone system testing at our center, we were unable to confirm a final diagnosis but did narrow down to two possibilities by following the algorithmic approach detailed above. We must always have a high index suspicion and should thoroughly evaluate patients coming to us with hypokalemia.
Conclusion
Patients presenting with hypokalemia should be evaluated for causes of hypokalemia. Simple tests like urinary electrolytes, urinary creatinine, and urine osmolality can be used to evaluate for secondary causes of hypokalemia. This can aid in establishing unusual causes of hypokalemia, missing out on which can be life threatening for the patient, who may end up developing life-threatening arrhythmia owing to recurring episodes of hypokalemia.
Here, we propose a possibility of one of the rare causes of hypokalemia, a possibility of Liddle-Gitelman overlap syndrome in a patient having features common to both. Further case reports and genetic studies are required to throw light on such possibility.
Authors' Contribution
N.A. did data collection and manuscript draft preparation. V.G. contributed in conceptualization and review of manuscript. S.N. and A.A. did the critical review of manuscript.
Conflict of Interest
None.
References
- Clinical and biochemical spectrum of hypokalemic paralysis in Northeast India. Ann Indian Acad Neurol. 2013;16(02):211-217.
- [CrossRef] [PubMed] [Google Scholar]
- Liddle's syndrome presenting with periodic paralysis. J Assoc Physicians India. 2009;57:481-482.
- [Google Scholar]
- Etiological search and epidemiological profile in patients presenting with hypokalemic paresis: an observational study. Indian J Endocrinol Metab. 2018;22(03):397-404.
- [CrossRef] [PubMed] [Google Scholar]
- Spectrum of hypokalemic paralysis from a tertiary care center in India. Indian J Nephrol. 2018;28(05):365-369.
- [CrossRef] [PubMed] [Google Scholar]
- Fluid and electrolyte disturbances. Harrison's Principles of Internal Medicine 2022:351.
- [Google Scholar]





