Translate this page into:
The potential of various herbal plants for potential therapeutic treatment for diabetes management
*Corresponding author: Prof. Sheelendra M Bhatt, PHD, Department of Biotechnology, IIMT University, Meerut, Uttar Pradesh, India. drsmbhatt@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhatt SM, Sharma N. The potential of various herbal plants for potential therapeutic treatment for diabetes management. Ann Natl Acad Med Sci (India). doi: 10.25259/ANAMS_70_2024
Abstract
This review is an attempt to cover a few useful herbal plants, like bitter melon, fenugreek, ginseng, cinnamon, garlic, and gymnema, beneficial in managing blood glucose levels due to saponin, terpenoids, flavonoids, and more which may be useful for controlling diabetes. We also cover a detailed description of gymnema, also known as gurmarin. In this review, we discuss possible mechanisms on how gurmarin helps manage diabetes by acting on taste bud receptors T1R1 and T1R3 and stimulating insulin to be released from both β-cells and islets, and also how PPARγ (peroxisome proliferator-activated receptor gamma) is activated to enhance insulin sensitivity where the release of insulin from β-cells is stimulated by gymnema. In addition, we discuss plant tissue culture methods to enhance gymnemic acid production.
Keywords
Blood sugar
Diabetes
GLUT-4 receptors
Gymnema
PPARγ
INTRODUCTION
For centuries, many plants have been used in traditional medicine systems such as Ayurveda, traditional Chinese medicine, and Native American medicine to help manage blood glucose levels. Prolonged insulin resistance is commonly acknowledged as a causative factor in the onset of type 2 diabetes mellitus (DM 2). In the management of diabetes, conventional antidiabetic drugs are frequently prescribed. While they demonstrate less efficacy, they also bring about unavoidable side effects over organs like kidney, liver, and blood cells. Conversely, gymnema medicinal plants have the potential to serve as a good alternative reservoir of antidiabetic substances.1 Over time, scientists have discovered that various plants contain many bioactive compounds and hence are used as pharmaceutical drugs to treat diabetes.
Bitter melon (Momordica charantia [M. charantia]) is another plant used in curbing diabetes since a compound called charantin has shown to have hypoglycemic effects by increasing insulin sensitivity and glucose uptake in cells. Numerous animal studies and clinical trials have demonstrated the remarkable impact of M. charantia on diabetes. The findings from the research indicate that M. charantia can improve insulin sensitivity, repair damaged pancreas islet β-cells, and stimulate insulin secretion.2 Additionally, M. charantia can regulate intestinal flora, inhibit glucosidase and amylase, scavenge free radicals, enhance the activity of adenosine monophosphate-activated protein kinase, and increase the expression of peroxisome proliferator-activated receptors (PPARγ), thereby reducing hyperglycemia.
Moreover, it can also function as a glucagon-like peptide 1 receptor agonist and an 11-hydroxysteroid dehydrogenase type 1 inhibitor to exert hypoglycemic effects. Consequently, hyperglycemic rats experienced a significant decrease in blood glucose level by 31.64% and a notable increase in insulin level by 27.35% when administered with the highest dosage of 300 mg/kg whole fruit.
Fenugreek seeds, commonly known as methi in Hindi, are rich in fiber and a compound called trigonelline has been found to lower blood glucose levels by stimulating insulin secretion and improving insulin sensitivity.3 In uncontrolled diabetes, the regular administration of 30 g of fenugreek seeds in divided doses has been shown to effectively reduce glycosylated hemoglobin (HbA1c) levels. Further analysis of the fenugreek seed extracts revealed the presence of various phytochemical compounds. Trigonelline exhibits various beneficial properties such as hypoglycemic, hypolipidemic, neuroprotective, antimigraine, sedative, memory-enhancing, antibacterial, antiviral, and antitumor activities. Additionally, it has been proven to alleviate diabetic auditory neuropathy and platelet aggregation. Its possible mechanism of action involves influencing β-cell regeneration, insulin secretion, activities of enzymes associated with glucose metabolism, reactive oxygen species, axonal extension, and neuron excitability.
Flavonoids, a class of plant secondary metabolites known for their antioxidant properties, were identified in the extracts. These flavonoids contribute to the potential health benefits associated with fenugreek consumption. Terpenoids, another group of secondary metabolites found in the extracts, are known for their diverse biological activities. These compounds have been reported to possess antimicrobial, anti-inflammatory, and anticancer properties. The presence of terpenoids in fenugreek seed extracts suggests that they may contribute to the plant’s therapeutic potential.4 Phenols, a class of aromatic compounds, were also detected in the fenugreek seed extracts. Phenols are known for their antioxidant and anti-inflammatory properties, which may contribute to the health benefits associated with fenugreek consumption.
Proteins, essential macromolecules involved in various biological processes, were found in the fenugreek seed extracts. These proteins may play a role in the plant’s physiological functions and could potentially contribute to its therapeutic properties.
Saponins, another class of phytochemicals identified in the extracts, have been reported to possess various biological activities, including antimicrobial, anti-inflammatory, and anticancer properties. The presence of saponins in fenugreek seed extracts suggests that they may contribute to the plant’s potential health benefits.5 Tannins, a group of polyphenolic compounds, were also detected in the fenugreek seed extracts. Tannins are known for their antioxidant and antimicrobial properties and have been associated with various health benefits, including anti-inflammatory and anticancer effects.
Overall, the phytochemical analysis of fenugreek seed extracts revealed the presence of flavonoids, terpenoids, phenols, proteins, saponins, and tannins. These compounds contribute to the potential therapeutic properties of fenugreek and may explain its traditional use in various medicinal practices. Further research is needed to fully understand the specific roles and potential health benefits of these phytochemicals in fenugreek.
Nopal (Opuntia ficus indica), a type of cactus, is rich in fiber and antioxidants that can help regulate blood sugar levels and improve insulin sensitivity.6
Ginseng, a popular herb in traditional medicine, has been found to have antidiabetic effects by improving glucose metabolism and insulin secretion.7
Russian tarragon, cinnamon, psyllium, and garlic also have various bioactive compounds that have been studied for their potential antidiabetic effects. Russian tarragon, for instance, contains compounds that can enhance insulin sensitivity and improve glucose uptake in cells.8
Cinnamon has been shown to improve insulin sensitivity and reduce fasting blood glucose levels. Psyllium, a type of soluble fiber, can help regulate blood sugar levels by slowing down the absorption of glucose.9–13
Garlic, known for its numerous health benefits, has been found to have hypoglycemic effects by increasing insulin secretion and improving insulin sensitivity.
While these botanicals can be beneficial in managing blood glucose levels, it is important to note that they should not replace prescribed medications or medical advice. It is always recommended to consult with a healthcare professional before incorporating any new supplements or botanicals into your diabetes management plan.14 Garlic is known to decrease HbA1c level significantly after 13 weeks if used in a dose of 750 mg/day,15 thus helpful in decreasing DM 2.
Gymnema sylvestre R. Br.
Gymnema sylvestre (G. sylvestre) R. Br., a member of the Asclepiadaceae family, is a globally distributed herb. Its leaves [Figure 1] are extensively utilized in Indian proprietary medicines for managing DM 1 and 2 and acting as a diuretic Khan et al.16 (2019) [Figure 2].
- Plant of Gymenma sylvestria br. leaves.

- Source metabolic noncommunicable disease health report of India: The ICMR-INDIA B national cross-sectional study (ICMR-INDIAB-17) (thelancet.com). Source: https://pubmed.ncbi.nlm.nih.gov/37301218/
G. Sylvestre, also known as gurmar, has been traditionally used in Ayurvedic medicine for its potential to support healthy blood sugar levels and its antidiabetic properties.17 It is known for its multifaceted approach to maintaining healthy blood sugar and supporting pancreas function.17 The Sanskrit name for the plant is madhunashini, which is widely recognized for its ability to treat diabetes, and its main component is gurmarin. Studies suggest that it may help regulate blood sugar levels, promote weight loss, and improve insulin sensitivity.18 These additional properties make gurmarin a multifunctional peptide with significant potential in the field of nutrition and health. Gymnema is native to South China, Ryukyu Island, Southeast Asia, India, Sri Lanka, and Africa.
Gymnemic acid is a triterpenoid saponin and is the primary active compound responsible for the plant’s pharmacological effects, which is a secondary metabolite produced. Gymnemic acid is the main component of G. sylvestre.19
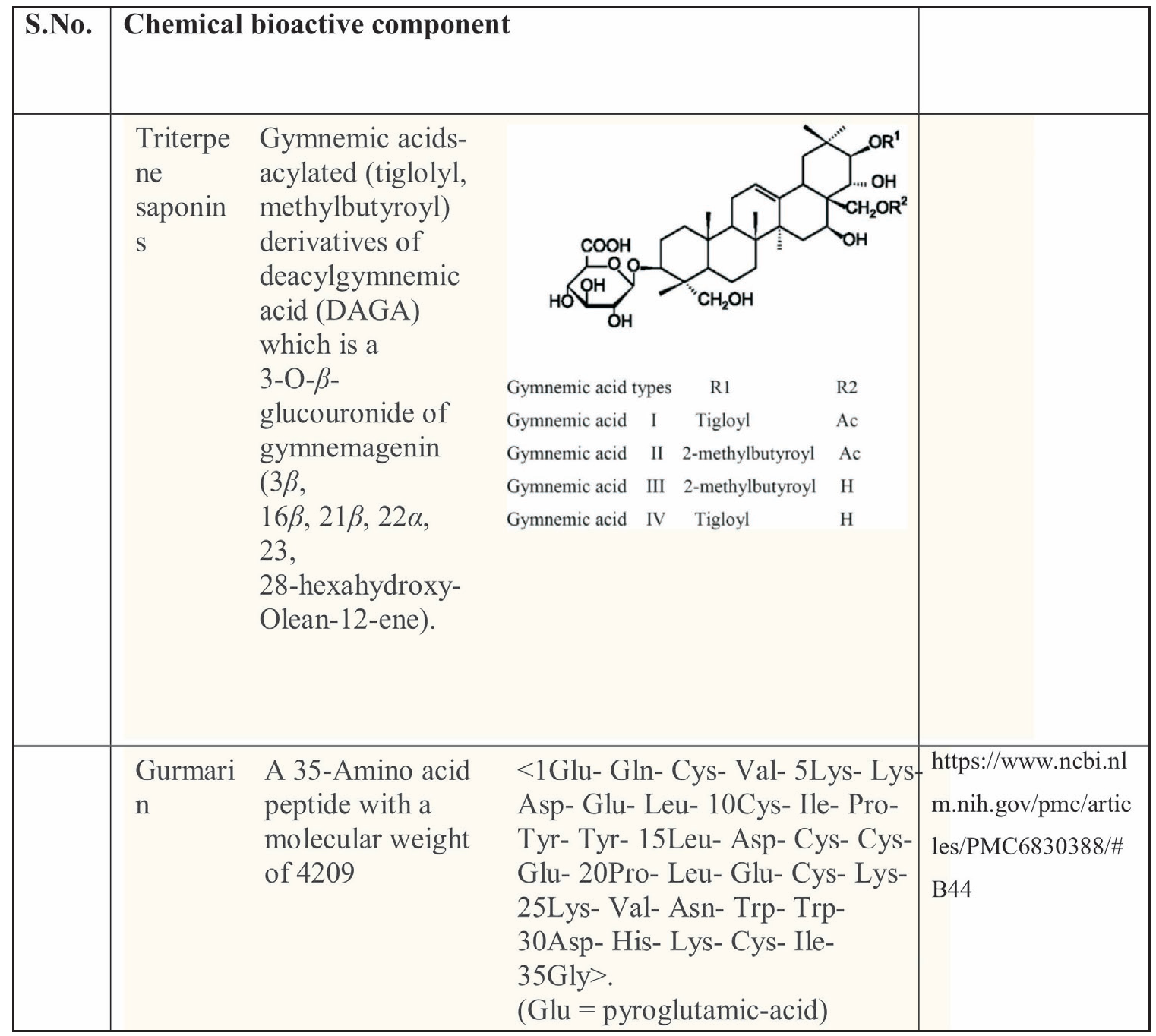
Gymnema, a medicinal plant native to India and Africa, is known for its unique set of nine closely associated acidic glycosides. These glycosides, including gymnemic acids A, B, C, and D, are the most significant compounds found in gymnema [Table 1].
| S.No. | Chemical bioactive component | ||
|---|---|---|---|
| Triterpene saponins | Gymnemic acids-acylated (tiglolyl, methylbutyroyl) derivatives of deacylgymnemic acid (DAGA) which is a 3-O-β-glucouronide of gymnemagenin (3β, 16β, 21β, 22α, 23, 28-hexahydroxy-Olean-12-ene). | ||
| Gurmarin | A 35-amino acid peptide with a molecular weight of 4209 < 1Glu- Gln- Cys- Val- 5Lys- Lys- Asp- Glu- Leu- 10Cys- Ile- Pro-Tyr- Tyr- 15Leu- Asp- Cys- Cys- Glu- 20Pro- Leu- Glu- Cys- Lys-25Lys- Val- Asn- Trp- Trp- 30Asp- His- Lys- Cys- Ile- 35Gly>. | (Glu = pyroglutamic-acid) | |
This secondary metabolite possesses various pharmacological properties, including nephroprotection, hypoglycemia, antioxidant, antimicrobial, and anti-inflammatory activities. Due to its minimal side effects and high efficacy in treating diabetes, gymnema has gained significant popularity in recent years and is one of the largest used herbal medicines for the cure of diabetes.20 Consequently, pharmaceutical companies have excessively exploited the plant’s biomass in the wild to extract gymnemic acid.
The bitter taste of G. sylvestre is due to the presence of saponin, which is triterpene glycoside that comes out when boiled in water. G. sylvestre triterpene saponins are known as gymnemic acids, gymnema saponins, and a polypeptide—gurmarin.21
Gurmarin from the leaves of Gymnema sylvestre
The peptide (gurmarin) derived from the leaves of G. sylvestre possesses an amino acid sequence that is responsible for its sweet-taste-suppressing properties. Gurmarin is a 35 amino acids residue polypeptide with an amino-terminal pyroglutamyl residue and has a molecular weight of 4,209 with three intramolecular disulfide bonds.22,23 This amino acid sequence of sweet-taste-suppressing peptide called gurmarin plays a crucial role in inhibiting the perception of sweetness, making gurmarin a promising natural alternative for individuals seeking to reduce their sugar intake or manage conditions related to excessive sugar consumption.
Gurmarin’s amino acid sequence consists of a specific arrangement of various amino acids, including glycine, alanine, proline, and cysteine, among others. These amino acids work synergistically to create a potent sweet-taste-suppressing effect. The precise arrangement and composition of these amino acids are essential for gurmarin’s ability to interact with taste receptors on the tongue, effectively blocking the perception of sweetness. When consumed, gurmarin interacts with the sweet taste receptors, known as T1R2 and T1R3, present in the taste buds. By binding to these receptors, gurmarin prevents the activation of the sweet taste pathway, thereby reducing the brain’s perception of sweetness.24 (Sivakumar & Bharathy, 2012). This mechanism effectively diminishes the desire for sugary foods and beverages, making it a valuable tool for individuals looking to curb their sugar cravings or manage conditions such as diabetes or obesity.
Gurmarin possesses a compact structure that includes an antiparallel β-hairpin (residues 22–34), multiple well-defined β-turns, and a cystine-knot motif commonly observed in toxic and inhibitory polypeptides. Furthermore, gurmarin’s amino acid sequence also contributes to its stability and bioavailability. The specific arrangement of amino acids allows gurmarin to withstand the harsh conditions of the digestive system, ensuring its intact delivery to the taste receptors in the mouth. This stability enhances its effectiveness and makes it a viable option for various applications, including the development of sweet-taste-suppressing additives or dietary supplements.
In addition to its sweet-taste-suppressing properties, gurmarin derived from G. sylvestre has also been found to possess other potential health benefits.
Gymnema leaves as antidiabetic therapeutics
These glucose-lowering effects are believed to be attributed to increased insulin secretion. Additionally, a methanol extract of G. Sylvestre leaf and callus has shown promising antidiabetic activities by regenerating β-cells. Although there is limited research on the specific active agents within the leaf extracts, aqueous ethanolic extractions have yielded two potentially active fractions. One fraction contains conduritol A, an acid-soluble polyol-polyhydroxyl cyclic compound, while the other fraction contains a mixture of acid-insoluble triterpenoid saponins (glycemic acids) known as GS3 and GS4.
The gymnema leaf extract possesses various properties such as laxative, diuretic, and cough suppressant. However, these effects may be considered unfavorable when the extract is used for its intended purpose of lowering glucose levels in diabetes patients. Additionally, the peptide “Gurmarin” found in the extract has been discovered to disrupt the taste buds’ ability to detect sweet and bitter flavors.
Recent reports suggest that gymnemic acid formulations can be effective against obesity due to their ability to delay glucose absorption in the blood. This is achieved by the gymnemic acid molecules filling the receptor locations on the taste buds and absorptive external layers of the intestine, preventing activation by sugar molecules present in food and absorption of sugar molecules by the intestine, respectively. The atomic arrangement of gymnemic acid molecules is similar to that of glucose molecules, which allows them to curb sugar cravings and result in lower blood sugar levels.
Gymnemic acid, a bioactive compound found in G. sylvestre, has been extensively studied for its antidiabetic properties. The main chemical constituents of G. sylvestre are a group of triterpenoid saponins known as gymnemic acids, which are considered to be the active compounds responsible for the antidiabetic effects of the extracts.25 These gymnemic acids have been found to stimulate insulin release and synthesis, improve glucose tolerance, and have anti-inflammatory activities. Additionally, they have been shown to have antidiabetic, antilipidemic, and anti-inflammatory effects.26 Furthermore, gymnemic acids have been reported to inhibit the intestinal absorption of glucose and oleic acid.27 Studies have also demonstrated the immunomodulatory properties of gymnemic acid, stimulating lymphocyte proliferation.
The presence of gymnemagenin and gymnemic acids in G. sylvestre extract has been recognized as being responsible for its antihyperglycemic effect. Furthermore, gymnemic acid, a saponin of triterpene glycoside contained in the leaves of G. sylvestre, has been found to possess potent antidiabetic properties. Additionally, extracts of G. sylvestre have been shown to stimulate insulin release in vitro by increased membrane permeability, indicating therapeutic potential for the treatment of noninsulin-dependent DM.
The 35 amino acids peptide gurmarin is a significant component found in G. sylvestre extract, known for its sugar suppression activity. This peptide has been shown to adhere to bitter and sweet taste receptors, temporarily inhibiting taste and thereby lowering sweet cravings. Additionally, gurmarin has been found to inhibit sweetener-mediated calcium responses of cells expressing the sweet taste receptor protein, T1R1/T1R3, thereby modulating sugar-feeding behavior. Furthermore, gurmarin has been demonstrated to depress taste responses to sugars and saccharin sodium, indicating its potential to inhibit sweet taste. Moreover, gurmarin has been reported to prevent the absorption of sugary foods, thus contributing to glycemic control in type 2 diabetes patients.
The presence of gurmarin in G. sylvestre extract aligns with its traditional use in managing type 2 diabetes, as it contributes to the suppression of sweetness and sugar absorption. This supports the findings that G. sylvestre extract helps promote weight loss and controls blood sugar levels. The inhibitory effect of gurmarin on sweet taste receptors and its ability to modulate sugar-feeding behavior makes it a promising candidate for further research in the management of diabetes and obesity.
Gymnemic acid, a mixture of triterpene glycosides extracted from the leaves of G. sylvestre, has been the subject of extensive research due to its potential pharmacological properties. The leaves of G. sylvestre contain triterpene saponins belonging to the oleanane and dammarane classes Tiwari et al.28,29 (2015). These saponins, including gymnemic acid, have been found to possess normoglycemic and hypolipidemic activity, stimulating insulin secretion without compromising β-cell viability.30 Additionally, G. sylvestre leaf extract has been shown to have immunomodulatory effects with a significant gymnemic acid content. Furthermore, in an animal study, G. sylvestre leaf extract improved serum cholesterol and triglyceride levels through the influence of lipid metabolism.31
The isolated triterpene glycoside fraction from G. sylvestre has been investigated for its potential blood glucose control benefits using in vitro methods.32 Moreover, G. sylvestre has been found to contain more than 20 saponin glycosides, including gymnemic acid, and has been shown to possess antidiabetic and antioxidant. Gymnemic acid, as an active compound in G. sylvestre, has been reported to have beneficial effects on vascular architecture and the expression of vascular endothelial growth factor in the diabetic rat kidney.33 Furthermore, gymnemic acid has been isolated and characterized as a mixture of triterpene glycosides, including gymnemic acid I, IV, VII, and gymnemagenin, which have shown promise in diabetic treatment.34
Liu et al.35 (2009) conducted a study to investigate the impact of an alcoholic extract of G. sylvestre (GS4) on insulin secretion in islets of Langerhans and various pancreatic β-cell lines. The results revealed that GS4 effectively stimulated insulin release from both β-cells of islets even without any additional stimulus. However, when 1mM EGTA was present, the GS4-induced insulin secretion was found to be inhibited.36
The suppression of sweet taste sensations in humans has also been attributed to gymnemic acids, which are a mixture of triterpene glycosides isolated from G. sylvestre. Additionally, various gymnemic acids, including GA I to GA XVIII, have been reported from the leaves of G. sylvestre.32 Furthermore, gymnemic acids have been found to have antidiabetic, anti-sweetener, and anti-inflammatory activities.37 The inhibitory potential of carbohydrate hydrolyzing enzymes and antioxidant activities of G. sylvestre methanol leaf extract has also been investigated.
SIGNALING REGULATION IN SUGAR METABOLISM
When PPARγ is activated in individuals with type 2 diabetes, it results in a notable improvement in various insulin and glucose parameters. This improvement is primarily attributed to the enhancement of overall insulin sensitivity throughout the body.29
Insulin sensitivity refers to the body’s ability to effectively respond to and utilize insulin, a hormone responsible for regulating blood sugar levels. In individuals with type 2 diabetes, insulin sensitivity is often impaired, leading to difficulties in maintaining normal blood glucose levels. However, when PPARγ is activated, it triggers a cascade of molecular events that help restore and enhance insulin sensitivity.
One of the key effects of PPARγ activation is an increase in the expression of genes involved in glucose metabolism and insulin signaling. This leads to improved insulin sensitivity in various tissues, including skeletal muscle, liver, and adipose tissue. As a result, these tissues become more responsive to insulin, allowing for better glucose uptake and utilization.
Furthermore, PPARγ activation also promotes the differentiation and maturation of adipocytes, or fat cells. This is significant because adipose tissue plays a crucial role in regulating glucose and lipid metabolism. By increasing the number and functionality of adipocytes, PPARγ activation helps to improve insulin sensitivity and reduce excessive fat accumulation, which is often associated with insulin resistance in individuals with type 2 diabetes.
Additionally, PPARγ activation has been shown to enhance insulin secretion from pancreatic beta cells, which are responsible for producing and releasing insulin. This further contributes to the overall improvement in insulin and glucose parameters in individuals with type 2 diabetes.
Overall, the activation of PPARγ in individuals with type 2 diabetes has a profound impact on insulin sensitivity throughout the body. By enhancing glucose metabolism, promoting adipocyte function, and improving insulin secretion, PPARγ activation leads to significant improvements in insulin and glucose parameters. This not only helps individuals with type 2 diabetes better manage their blood sugar levels but also has the potential to alleviate the underlying insulin resistance associated with the condition.
The treatment of diabetes often involves the use of herbal products and secondary metabolites derived from traditional medicinal plants. These substances have shown potential in regulating insulin signaling pathways, facilitating the movement of glucose transporter type 4 receptors, and activating the PPARγ, all of which are crucial for glucose metabolism and regulation.8
One important mechanism by which herbal products and secondary metabolites can aid in diabetes treatment is by inhibiting glucose absorption. Certain flavonoids found in these natural products have been found to block intestinal α-amylase and α-glucosidase enzymes, which are responsible for breaking down complex carbohydrates into glucose. By inhibiting these enzymes, flavonoids can reduce the amount of glucose that is absorbed into the bloodstream, helping to control blood sugar levels.8
However, it is important to note that while herbal products and secondary metabolites show promise in diabetes treatment, thorough studies are necessary to validate their effectiveness and safety. These studies should include both in vitro and in vivo experiments to assess the mechanisms of action and potential side effects of these natural compounds.
Furthermore, large-scale, well-designed clinical trials are essential before recommending the use of herbal products and secondary metabolites for the treatment and prevention of diabetes. These trials should involve a diverse population of individuals with diabetes and should assess the long-term effects of these natural preparations on blood sugar control, insulin sensitivity, and overall health.
TOXICOLOGICAL AND SAFETY EVALUATION
The safety of numerous botanical herbs, including gymnema, has not undergone comprehensive evaluation, raising concerns about their potential risks and side effects. While these herbs have been used for centuries in traditional medicine practices, their safety profiles remain largely unknown due to limited scientific research and regulatory oversight.38
There have been some findings from human studies suggesting that specific gymnema extracts could potentially amplify the glucose-lowering effects of certain antidiabetic medications. However, due to the uncertainties surrounding the composition of various gymnema preparations, potential interactions between herbs and drugs as well as the indications of glucose-lowering or hypoglycemic effects, it is important to acknowledge that the use of gymnema-based dietary supplements alongside authorized antidiabetic drugs may carry certain risks.
Hepatotoxicity of Gymnema
Hepatotoxicity of cisplatin and the role played by gymnema in saving liver from various toxins is induced after cisplatin injection. Gymnema can save from hepatotoxicity due to its antioxidant-rich properties and other properties. As a result, serum liver function biomarkers (alanine transaminase, aspartate transaminase, and total bilirubin), were reduced drastically. Some workers had studied paracetamol-induced hepatorenal toxicity, where the powder of G. sylvestre leaves was supplemented with rosemerry powder, reduced toxicity drastically Elmetwally et al.39 (2024).
Type 2 diabetes mellitus: Clinical trail
In a randomized trial, patients with DM 2 can benefit from the combined administration of inositols, α-lactalbumin, G. sylvestre, and zinc, as it has been shown to enhance their lipid metabolic profile.38,40,41 Baskaran et al.41 (1990) in their study revealed that the patients showed a remarkable decrease in their blood glucose levels as well as in the levels of HbA1c and glycosylated plasma proteins. Among the 22 diabetic patients who participated in the study, five individuals were able to completely stop taking their conventional medication. These patients were able to maintain their blood glucose balance solely by using aqueous leaf extract of G. sylvestre containing mainly gymnemic acid (GS4).
This outcome is particularly significant as it demonstrates the effectiveness of GS4 in managing diabetes and its potential to replace or reduce the reliance on conventional drugs. The fact that these patients were able to maintain their blood glucose levels without the need for additional medication highlights the potential benefits of GS4 as a standalone treatment option.
The ability of GS4 to effectively regulate blood glucose levels, as evidenced by the decline in HbA1c and glycosylated plasma proteins, suggests that it may have a positive impact on the overall diabetes management. This could potentially lead to a decrease in the dosage of conventional drugs required, reducing the risk of side effects and improving the overall well-being of DM 2 patients.42
Tissue culture techniques
Certain tissue culture techniques also are useful for enhanced production of gymnemic acid production. In shoot culture, gymnemic acid addition of certain components is reported by Zimare et al.43 (2020). Gymnemic acid production is an important constituent in antidiabetic function; therefore, as per classification, gymnemic acid is part of oleanane type of triterpenoid saponin.
Many elicitors have been used to enhance the gymnemic acid production continuously throughout the year in suspension culture.44 As per the report, the response elicited by A. niger, which was the highest, measured at 98.65 ± 0.93 mg/g dry cell weight (gDCW), exhibiting an impressive 11.2-fold increase, while in the abiotic phase at a concentration of 2 m and after 24 hours, CdCl2 exhibited the highest response, reaching 59.97 mg/gDCW.45 In suspension culture, mostly callus is induced in MS media in proper ratio of IAA (indole acetic acid and BA (benzyl adenine).46
FORMULATION FOR DIABETES TREATMENTS DIABETES TREATMENTS
Herbal medicine has gained attention for its potential glycemic control, with G. sylvestre being recognized for its antidiabetic properties Kahksha et al.47 (2022). Previous research has demonstrated the efficacy of G. sylvestre in reducing blood sugar levels and alleviating diabetes-related symptoms. However, challenges persist in achieving standardized formulations that ensure consistent therapeutic outcomes. The formulation aims to offer a safe, effective, and easily administrable solution for individuals dealing with diabetes. The need for such an invention is evident in the limitations of existing interventions, emphasizing the significance of a standardized herbal remedy with proven efficacy and minimal side effects. The unique composition and methodology of the formulation represent a promising advancement in the pursuit of holistic glycemic control solutions, contributing to the overall well-being of individuals grappling with diabetes and related health concerns.
An herbal formulation Gymnema Gold Plus formulated and patented in capsule form for the regulation of blood glucose levels in individuals experiencing high glycemic index, as shown in Figures 1–3.16
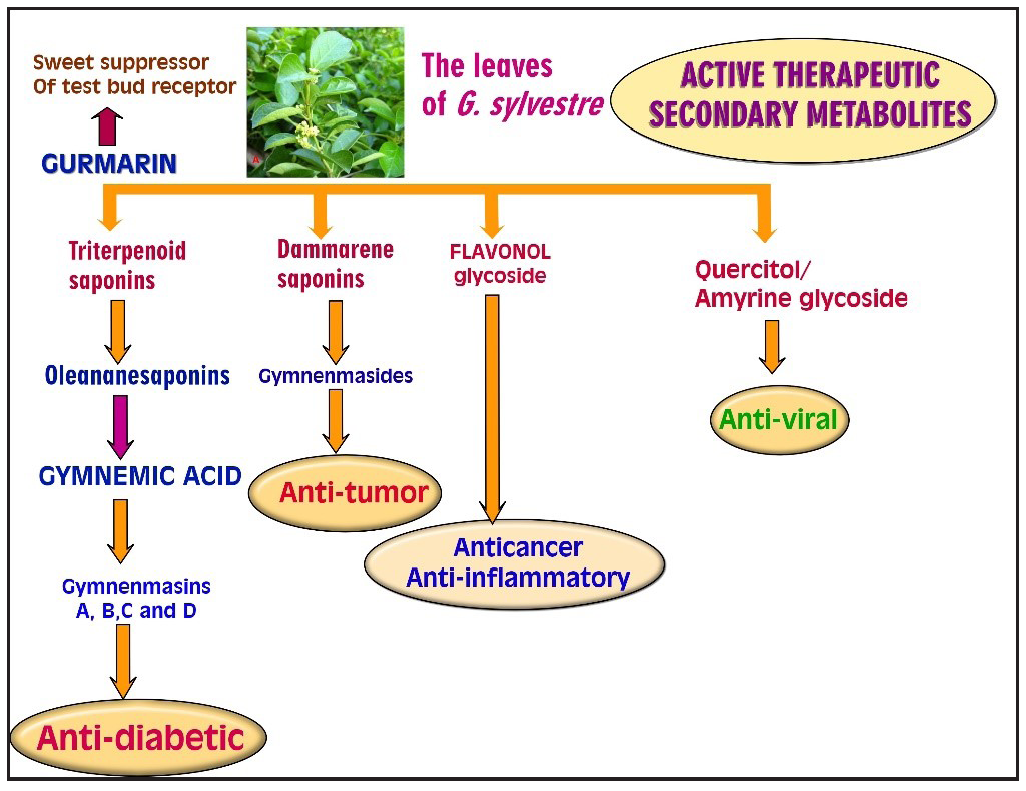
- Therapeutic effect of different secondary metabolites present in gymnema. Source: https://pmc.ncbi.nlm.nih.gov/articles/PMC6830388/
In light of the above, G. sylvestre R. Br. powder extract in the range of 70–80% of the herbal formulation, with moringa powder in the range of 10–20% of the herbal formulation, and fenugreek seeds powder in the range of 10% and 10% Withania somnifera, aka Ashwagandha, of the herbal formulation in a specific ratio of 3:1:1:1. Each oral dosage form includes 2.5 gm of gymnema powder, 0.25 gm of moringa powder, 0.25gm of fenugreek seeds, and 0.25gm of Withania somnifera [Figures 4-5].

- (a) Three-dimensional structure of gurmarin and gymnemic acid (source NCBI) (b) Gurmarin 3 d structure (https://www.rcsb.org/structure/1c4e).

- Class of tri-terpenoid saponin. Source: https://pmc.ncbi.nlm.nih.gov/articles/PMC6830388/
Method for preparation
Formulation for glycemic control includes the following steps: (1) washing G. sylvestre leaves with distilled water to remove dirt, washing the above-washed leaves with mild soap solution, rinsing the leaves thrice with distilled water, blot-drying the leaves with tissue paper, shade-drying the leaves at room temperature for two weeks, and cutting the dried leaves to small pieces, and (2) powdering the cut leaves in a mixer, sieving the powdered leaves using a 20 μm mesh sieve to obtain a uniform size range, adding moringa powder in the range of 10–20% of the formulation into the above-powdered extract of G. sylvestre leaves along with fenugreek seed powder in the range of 10% of the formulation, and formulating the above mixture in an oral dosage form.
Clinical trial
The resulting herbal formulation is a recommended daily dosage of 3 g per one spoon in 60 patients having diabetic conditions. The method of administration involves dissolving the powder in boiling water, filtering after five minutes, and oral consumption on an empty stomach. This regimen is advised to be undertaken without any other medications or drinks for 60 days.
The primary endpoints for the study included changes in diabetic panel parameters, such as:
-
Fasting Blood Sugar (FBS)
-
Postprandial Blood Sugar (PBS)
-
Glycosylated Hemoglobin (HbA1c)
Statistical analysis
All patients in the study with relevant safety and efficacy data were considered for the analysis. Efficacy and safety endpoints were analyzed for the relevant study population. A descriptive analysis of demographic characteristics was performed. Mean and standard deviation was derived for numeric and categorical parameters. Vital signs at each visit were also analyzed descriptively (data not shown here).
Gymnema Gold Plus was given for 60 days, leading to statistically significant changes in sugar fasting [Figures 6, 7 and 8] HbA1c (p < 0.001), FBS (p < 0.001), and PBS (p < 0.001) for both prediabetic and newly diagnosed diabetic patients.16
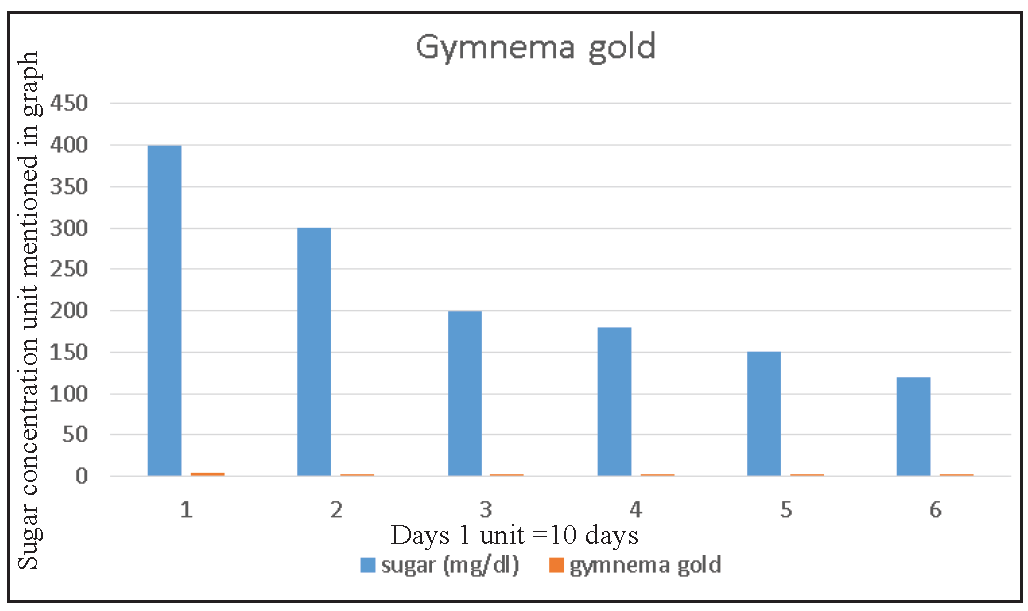
- Illustrates that basic reduction in sugar level after taking gymnema powder of 2, 3, and 4 g (orange color) consumption, which results in blood sugar level reduction from 400 to 120 mg/dL after 60 days (1 = 10 days) (data shown means and SD ± 0.25). Source: https://pubmed.ncbi.nlm.nih.gov/28459647/

- Illustrates that sugar fasting levels (orange line) decrease after taking the herbal formulation, reaching around 120 mg/dL after six days from 400 mg/dL sugar level, compared to sugar levels (blue line) which remain around 120 mg/dL (data shown means and SD ± 0.15) SD: Standard deviation. Source: https://pmc.ncbi.nlm.nih.gov/articles/PMC6830388/

- Illustrates reduction in HbA1c level during consumption of Gymnema Gold Plus after 60 days (blue color). High HbAc level was 7.8 without gymnema, whereas after 60 days, the level was only normal in the range of 5.5 (orange color), which means that blood sugar has been controlled effectively by the consumption of Gymnema Gold Plus formulation (data shown means and SD ± 0.15). HbA1c: Glycated hemoglobin.
The blue bar graph shows days and the orange bar graph shows the blood sugar levels of someone who is taking the herbal formulation. The line starts at around 400 mg/dL and stays relatively up to 120–180 mg/dL level achieved in six days with the consumption of gymnema, which remains through the 60 days, depending on age. This means that the person’s blood sugar levels are not changing much over time after consumption of the herbal formulation.
The difference between the two lines shows that the herbal formulation helps to lower blood sugar levels. Surprisingly, formulations comprising Ashwagandha, moringa powder, fenugreek seed powder, and G. sylvestre extract provide a synergistic effect in diabetic patients, especially in type 2 diabetic patients, with respect to, amongst others, glycemic (blood sugar) control by inducing insulin secretion [Figures 7 and 8].
CONCLUSION
Several beneficial herbal plants, such as M. charantia, fenugreek, ginseng, cinnamon, garlic, and gymnema, are useful in managing blood glucose levels. This is due to the presence of compounds like saponin, terpenoids, flavonoids, and others, which have potential benefits for controlling diabetes. In this context, let’s delve into a detailed description of gymnema, also known as gurmarin.
Gymnema has been found to play a significant role in curbing diabetes. It achieves this by acting on taste bud receptors: taste type 1 receptor 2 and taste type 1 receptor 3 (T1R2/T1R3), which, in turn, stimulate the release of insulin from both β-cells of islets. Additionally, gymnema activates PPARγ, a receptor that enhances insulin sensitivity.
Acknowledgment
The author is dedicating this work to all those who supported directly and indirectly during the writing of the manuscript.
Authors’ contributions
SMB: Draft, concept and review; NS: Editing and review.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- The effect of a 14-day gymnema sylvestre intervention to reduce sugar cravings in adults. Nutrients. 2022;14:5287.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of administration of fenugreek seeds on HbA1c levels in uncontrolled diabetes mellitus – A randomized controlled trial. Int J PharmTech Res. 2015;8:180-2.
- [Google Scholar]
- Exploring the therapeutic ability of fenugreek against type 2 diabetes and breast cancer employing molecular docking and molecular dynamics simulations. Evid Based Complement Alternat Med. 2018;2018:1943203.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of Fenugreek (Trigonella Foenum-Graecum L.) intake on glycemia: A meta-analysis of clinical trials. Nutr J. 2014;13:7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The effect of nopal (Opuntia ficus indica) on postprandial blood glucose, incretins, and antioxidant activity in Mexican patients with type 2 diabetes after consumption of two different composition breakfasts. J Acad Nutr Diet. 2014;114:1811-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical potentials of ginseng polysaccharide for treating gestational diabetes mellitus. World J Clin Cases. 2021;9:4959-79.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An overview of herbal products and secondary metabolites used for management of type two diabetes. Frontiers in Pharmacology; 2017.
- Cinnamon for Diabetes Mellitus. Cochrane Database of Systematic Reviews; 2012.
- Ceylon Cinnamon: A Versatile Ingredient for Futuristic Diabetes Management. Journal of Future Foods. 2022:125-42.
- [CrossRef] [Google Scholar]
- The Effects of Cinnamon on Diabetes Mellitus: A Narrative Review. Int. Electron. J. Med. 2019
- [CrossRef] [Google Scholar]
- Efficacy of cinnamon in patients with type II diabetes mellitus: A randomized controlled clinical trial. Clin Nutr. 2019;38:549-56.
- [CrossRef] [PubMed] [Google Scholar]
- Cinnamon as a Complementary Therapeutic Approach for Dysglycemia and Dyslipidemia Control in Type 2 Diabetes Mellitus and Its Molecular Mechanism of Action: A Review. Nutrients. 2022;14:2773.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence and Patterns of Herbal Medicine Use among Type 2 Diabetes Mellitus Patients at the University Teaching Hospitals in Lusaka. J. Biomed. Res. Environ. Sci.. 2022;3(1):074-81.
- [CrossRef] [Google Scholar]
- Effects of Garlic (Allium Sativum) on Blood Glucose Level in Type 2 Diabetes Mellitus Patients Treated with Metformin. J. Enam Med. Coll.. 2017;7(3):151-155.
- [CrossRef] [Google Scholar]
- Comprehensive review on phytochemicals, pharmacological and clinical potentials of Gymnema sylvestre. Front Pharmacol. 2019;10:1223.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Metabolic non-communicable disease health report of India: The ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17) Lancet Diabetes Endocrinol. 2023;11:474-89.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Gymnema Sylvestre in the Control of Diabetes: A Review. International Journal of Pharmaceutical Quality Assurance. 2023:214-219.
- [Google Scholar]
- Gymnema Sylvestre-a Review. Indian Journal of Forensic Medicine and Toxicology. 2020:1081-1084.
- [Google Scholar]
- Three-dimensional structure of gurmarin, a sweet taste-suppressing polypeptide. J Biomol NMR. 1995;5:297-305.
- [CrossRef] [PubMed] [Google Scholar]
- Amino acid sequence of sweet-taste-suppressing peptide (gurmarin) from the leaves of Gymnema sylvestre. J Biochem. 1992;111:109-12.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular mechanism of interaction between human sweet taste receptors and antidiabetic agents of Gymnema sylvestre through docking studies. International Journal of Research in Phytochemistry & Pharmacology. 2012;2(4):164-70.
- [PubMed] [Google Scholar]
- Gymnema Sylvestre (Gurmar): A Potent Herb with Anti-Diabetic and Antioxidant Potential. Pharmacognosy Journal. 2019:201-6.
- [CrossRef] [Google Scholar]
- Pharmacognostical Profile of Gymnema Sylvestre and Its Anti-Hyperglycemic Activity. J. Pharm. Res. Int. 2021:365-76.
- [CrossRef] [Google Scholar]
- Two new triterpenoid saponins from Gymnema sylvestre. J Integr Plant Biol. 2008;50:589-92.
- [CrossRef] [PubMed] [Google Scholar]
- Structure Activity Relationship Studies of Gymnemic Acid Analogues for Antidiabetic Activity Targeting PPARγ. Curr Comput Aided Drug Des. 2015;11:57-71.
- [CrossRef] [PubMed] [Google Scholar]
- Cross-Talk between PPARgamma and Insulin Signaling and Modulation of Insulin Sensitivity. PPAR Res. 2009;2009:818945.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A novel dihydroxy gymnemic triacetate isolated from Gymnema sylvestre possessing normoglycemic and hypolipidemic activity on STZ-induced diabetic rats. J Ethnopharmacol. 2009;126:339-44.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of administration with the extract of Gymnema sylvestre R. Br leaves on lipid metabolism in rats. Biol Pharm Bull. 2001;24:713-7.
- [CrossRef] [PubMed] [Google Scholar]
- In Vitro Antidiabetic Effects of Isolated Triterpene Glycoside Fraction from Gymnema sylvestre. Evid Based Complement Alternat Med. 2018;2018:7154702.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Beneficial Effects of Gymnemic Acid on Three-Dimensional Vascular Architecture and Expression of Vascular Endothelial Growth Factor of Intrarenal Segmental and Interlobar Arteries in Diabetic Rat Kidney. Funct. Foods Heal. Dis.. 2022;12(6):340-51.
- [CrossRef] [Google Scholar]
- In silico evaluation of naturally isolated triterpene glycosides (TG) from Gymnema sylvestre towards diabetic treatment. Heliyon. 2021;7:e08407.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Characterisation of the insulinotropic activity of an aqueous extract of Gymnema sylvestre in mouse beta-cells and human islets of Langerhans. Cell Physiol Biochem. 2009;23:125-32.
- [CrossRef] [PubMed] [Google Scholar]
- Antisweet Natural Products: V: 1) Structures of Gymnemic Acids Viii—Xii From Gymnema Sylvestre r: Br. Chem. Pharm. Bull.. 1992;40(7):1779-82.
- [Google Scholar]
- “Hypoglycaemic effect of alcoholic extracts of the leaves of abroma augusta & gymnema sylvestre plants in type ii diabetes mellitus patients”. Indian Journal of Public Health Research & Development. 2020;11(7):288-294.
- [PubMed] [Google Scholar]
- Risk assessment of substances used in food supplements: The example of the botanical Gymnema sylvestre. EFSA J. 2018;16:e16083.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Protective Effect of Gymnema Sylvestre and Rosmarinus Officinalis Leaves Against Hepatorenal Toxicity of Paracetamol in Experimental Rats. 2024;10(2):407-31.
- Combined Inositols, α-Lactalbumin, Gymnema Sylvestre and Zinc Improve the Lipid Metabolic Profile of Patients with Type 2 Diabetes Mellitus: A Randomized Clinical Trial. J Clin Med. 2023;12:7650.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antidiabetic effect of a leaf extract from Gymnema sylvestre in non-insulin-dependent diabetes mellitus patients. J Ethnopharmacol. 1990;30:295-300.
- [CrossRef] [PubMed] [Google Scholar]
- Modulation of Insulin Resistance by Silybum marianum Leaves, and its Synergistic Efficacy with Gymnema sylvestre, Momordica charantia, Trigonella-foenum graecum Against Protein Tyrosine Phosphatase 1B. Biotechnol Genet Eng Rev 2023:1-23.
- [Google Scholar]
- “Potential mevalonate pathway precursors for enhanced production of gymnemic acid”. Indian Journal of Experimental Biology (IJEB). 2020;58(09):651-655.
- [Google Scholar]
- Improved Gymnemic Acid Production in the Suspension Cultures of Gymnema Sylvestre through Biotic Elicitation. Plant Biotechnol. Rep.. 2013;7(4):519-25.
- [CrossRef] [Google Scholar]
- Abiotic elicitation of gymnemic acid in the suspension cultures of Gymnema sylvestre. World J Microbiol Biotechnol. 2012;28:741-7.
- [CrossRef] [PubMed] [Google Scholar]
- Gymnemic Acids: Sources, Properties, and Biotechnological Production. In Plant-derived Bioactives: Production, Properties and Therapeutic Applications 2020:177-93.
- [CrossRef] [Google Scholar]
- Recent developments made in the assessment of the antidiabetic potential of gymnema species - From 2016 to 2020. J Ethnopharmacol. 2022;286:114908.
- [CrossRef] [PubMed] [Google Scholar]





