Translate this page into:
A comparative study on clinical features and COVID-19 severity in pediatric patients and adults
*Corresponding author: Sailu Yellaboina, Department of Biochemistry, All India Institute of Medical Sciences, Hyderabad, Telangana, India. bio.sailu@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singh MS, Singh T, Ansari MA, Yellaboina S. A comparative study on clinical features and COVID-19 severity in pediatric patients and adults. Ann Natl Acad Med Sci (India). 2024;60:172-7. doi: 10.25259/ANAMS_1078_2023
Abstract
Introduction
Coronavirus disease (COVID-19) has emerged as a global threat affecting diverse populations. While the severity of the disease is often associated with comorbidities and inflammation, pediatric patients generally experience a milder course. Limited studies exist regarding the factors influencing acute COVID-19 severity in pediatric patients compared to the elderly. This review aims to bridge this knowledge gap by investigating clinical presentations and potential factors contributing to severity, providing valuable insights into the relationship between age and disease outcomes.
Methods
Our study encompassed diverse settings, including hospitals and communities, with a focus on children and adults. We conducted a comprehensive literature review, analyzing PCR and laboratory-confirmed COVID-19 cases. The primary purpose was to elucidate demographic and clinical features, shedding light on the prevalence of symptoms and signs in pediatric patients.
Results
The results demonstrate a reduced prevalence of COVID-19 in hospitalized children as compared to adults, that are consistent with the generally milder clinical trajectory observed in pediatric cases. The study identifies factors contributing to severity in different age groups, emphasizing the need for a nuanced understanding of COVID-19 manifestations.
Conclusion
This literature review enhances our comprehension of COVID-19 across age groups, emphasizing the milder nature of the disease in pediatric patients. By uncovering factors influencing severity, this work contributes valuable knowledge for effective patient management, especially in older age groups where the risk is higher. The distinctive ability of younger individuals to combat the virus underscores the importance of tailored strategies for diverse age demographics in addressing this global health crisis.
Keywords
COVID-19
Coronavirus
SARS-CoV-2
Pediatric patients
Pediatric
Severity
Age
INTRODUCTION
Since the onset of 2019, the international community has been contending with the profound ramifications of the COVID-19 pandemic, presenting formidable challenges to healthcare systems worldwide. The causative agent, SARS-CoV-2, responsible for instigating COVID-19, was identified in respiratory specimens procured from individuals diagnosed with pneumonia and subsequently progressing to respiratory failure.1 The analyzed cases were stratified into symptomatic and asymptomatic categories, wherein the former exhibited clinical manifestations such as fever, rhinorrhea, influenza-like symptoms, and general malaise, necessitating subsequent hospitalization. Conversely, asymptomatic cases were characterized by the absence of discernible clinical signs or symptoms. It is crucial to underscore that the lack of symptomatic presentation did not function as a determinant for assessing the severity of the cases.
In light of the official declaration of COVID-19 as a pandemic, a plethora of cases delineating its clinical manifestations has come to the surface, with a substantial number involving pediatric patients necessitating hospitalization. Among individuals succumbing to COVID-19, a considerable proportion exhibited preexisting conditions such as hypertension, diabetes, and, in specific instances, cardiovascular diseases, potentially compromising their immune responses. However, the occurrence of these comorbidities was notably infrequent in the pediatric demographic, indicative of a more resilient immune response to the virus.2
Thrombosis and thrombocytopenia, which involve blood clots and low platelet counts, have been reported as rare adverse events following COVID-19 vaccination with COVISHIELD in India.3 The condition associated with these adverse events is known as vaccine-induced immune thrombotic thrombocytopenia (VITT) or thrombosis with thrombocytopenia syndrome (TTS). The exact cause of these adverse events is still under investigation, and no predisposing risk factors have been conclusively identified so far. However, it has been observed that antibodies that bind platelet factor 4, similar to those associated with heparin-induced thrombocytopenia, have been identified in some cases.4
Research findings consistently highlight a lower susceptibility of children and pediatric patients to severe outcomes from COVID-19. Both medical literature and an expanding repository of cases underscore a comparable infection incidence between adults and children. Nevertheless, the pediatric demographic tends to manifest milder disease forms, as evidenced by a low case fatality rate (CFR) of less than 0.1%. Furthermore, a case series encompassing four infants born to mothers with COVID-19 revealed that none of the three infants subjected to testing exhibited positive results for the virus or displayed clinical symptoms. This observation implies a minimal likelihood of vertical transmission of the virus.5
Due to the scarcity of comprehensive investigations into risk factors for COVID-19 in pediatric populations, our objective is to systematically collate and analyze extant data to discern and expound upon these factors. This endeavor seeks to offer invaluable insights to healthcare professionals tasked with mitigating the repercussions of viral infections in this demographic.
METHODS
The methodology for the systematic analysis was meticulously designed to ensure a comprehensive and rigorous review of the literature related to COVID-19 in neonates and children. The identification of relevant references commenced with a thorough search across reputable databases, including PubMed, NCBI, and Lancet’s EClinicalMedicine, utilizing specific and pertinent terms associated with 2019-nCoV, COVID-19, and SARS-CoV2 in the pediatric population. Notably, the focus was exclusively on articles published in English to maintain uniformity and accessibility.
The initial screening involved a meticulous review of 76 articles and consultation with 25 databases to ascertain a broad understanding of the existing literature. Subsequently, a judicious removal of duplicates was executed, resulting in 58 review articles and 20 datasets for further scrutiny. This refined dataset underwent a rigorous screening process, narrowing down to 40 review articles and 14 datasets that met the predefined inclusion criteria.
A stringent approach was adopted to maintain the quality and reliability of the selected studies. One particular study, which investigated infant cases, was excluded due to concerns about the data source’s reliability and incomplete parameters, ensuring the integrity of the final dataset. In addressing potential biases and limitations such as repeated calculations and missing data, a judicious decision was made to exclude this study from the pooled data, thereby enhancing the robustness of the systematic analysis.
The quantitative facet of the analysis benefited from the inclusion of seven meticulously selected case series specifically focused on pediatric COVID-19, facilitating a nuanced quantitative examination. Additionally, a qualitative perspective was enriched by the inclusion of 28 review papers, providing a comprehensive and detailed understanding of the subject matter as given in Figure 1.6-12
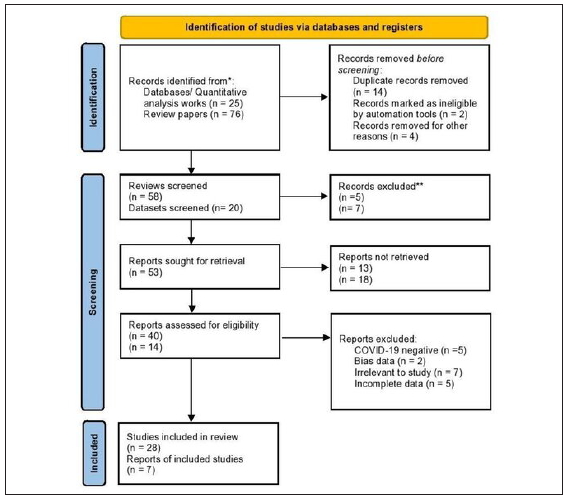
- Flowchart depicting the systematic process of study selection in the analysis.
RESULTS
In our examination of epidemiological and clinical features, the targeted population comprises individuals up to the age of 20, with gender deemed irrelevant to our study parameters. Noteworthy is the fact that 75% of the individuals under consideration had a documented history of household contact. Analysis of the provided table reveals that a substantial 98% of the scrutinized patients manifested mild to moderate disease severity, while only 1% encountered severe to critical disease conditions. It is particularly noteworthy that, among the 93 children enrolled in the study, a mere 2 necessitated intensive care, and only 1 child experienced complications. This unequivocally points to a diminished incidence of complications within the pediatric demographic, a finding underscored in the results section as given in Table 1.
| Studies referred to | Study A6 | Study B7 | Study C8 | Study D9 | Study E10 | Study F11 | Study G12 |
|---|---|---|---|---|---|---|---|
| Case number (n) | 6 | 15 | 10 | 20 | 31 | 6 | 5 |
| n / Total sample size | 6/93 | 15/93 | 10/93 | 20/93 | 31/93 | 6/93 | 5/93 |
| Median age (years) | 3 y | 7 y | 6.5 y | 2 y | 7 y | 7 y | 3 y |
| Age range | 1–7 y | 4–14 y | 3 m–10 y | 1 d–14 y | 6 m–17 y | 7 m–14 y | 10 m–6 y |
| Male (%) | 2 (33) | 5 (33) | 4 (40) | 13 (65) | 15 (48) | 5 (83) | 4 (86) |
| Disease severity | |||||||
| Mild | 2 | 3 | 6 | 4 | 17 | 3 | 2 |
| Moderate | 3 | 12 | 4 | 15 | 14 | 3 | 3 |
| Severe | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Critical | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Clinical features | |||||||
| Asymptomatic | 0 | 8 | 0 | 2 | 7 | 3 | 4 |
| Fever | 6 | 5 | 8 | 12 | 20 | 3 | 1 |
| Cough | 6 | 1 | 6 | 13 | 14 | 2 | 1 |
| GI symptoms | 4 | 0 | 0 | 3 | 3 | 1 | 0 |
| Outcomes | |||||||
| Intensive care | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Complication | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
The manifestations of COVID-19 encompassed a spectrum of presentations, ranging from asymptomatic cases to distinctive symptoms. Around 26% of the patient cohort manifested asymptomatic presentations, while 59% displayed pyrexia, representing a substantial portion of the overall study group. Cough was reported in approximately 46% of patients. However, there was a notable degree of variability in outcomes, with only a minority of patients manifesting gastrointestinal symptoms as given in Figure 2.
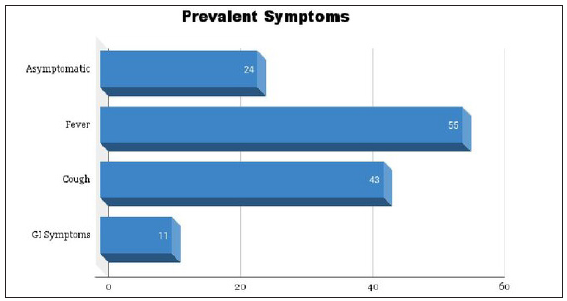
- Bar graph depicting the proportional distribution of symptoms associated with COVID-19.
The pathophysiological processes governing viral infection encompass coronaviruses, a class of single-stranded positive-sense ribonucleic acid (RNA) viruses characterized by distinctive spike-like projections on their surface.13 angiotensin-converting enzyme 2 (ACE2) receptors, distributed widely in various tissues throughout the human body, particularly on the cells of alveolar epithelium II, play a pivotal role in this process. The spike proteins that encapsulate the coronavirus predominantly bind to ACE2 receptors situated on type II alveolar cells, facilitating the introduction of the virus’ RNA into these cells. Consequently, this viral RNA takes command of the cell, compelling it to generate and release multiple copies of the virus into the alveoli. This cascade of events culminates in the lysis of the host cell, with progeny coronaviruses subsequently infecting adjacent cells. Significantly, this entire sequence may unfold asymptomatically in the host, underscoring its role as a pivotal factor in the asymptomatic transmission and efficient dissemination of COVID-19. The ascertainment of COVID-19 necessitates a meticulous clinical evaluation aligned with established guidelines, bolstered by supplementary insights gleaned from laboratory tests and radiological observations.14
The symptomatic expression of COVID-19 in neonates and children commonly presents as mild, demonstrating consistent patterns across diverse geographical locations.15,16 Hospitalization for children is predominantly prompted by symptoms including fever and respiratory complications, encompassing manifestations such as tussis, pharyngitis, facial erythema, rhinorrhea, rapid breathing or respiratory distress, and an elevated heart rate (tachycardia).17,18 Although infrequent, documented instances highlight the occurrence of neurological manifestations, involuntary muscle contractions, and altered mental status in some cases.19
COVID-19 severity classifications, spanning asymptomatic infections, and mild, moderate, severe, and critical cases, have been established through the integration of clinical features, laboratory test results, and X-ray imaging criteria.20 Notably, lymphocytopenia, a common occurrence in adults with severe COVID-19 and often associated with unfavorable outcomes, does not typically manifest in children, potentially attributed to the naturally higher percentage of lymphocytes in this age group.21,22 Conversely, adults exhibit significantly heightened levels of D-Dimer, ferritin, and coagulopathy, while these manifestations are infrequently observed in the pediatric population.23
Many of the observed laboratory abnormalities in children lack specificity. Notably, 69.2% of children exhibited leukocyte counts within the normal range, and instances of neutrophilia or neutropenia were infrequent, occurring in less than 5% of cases. Platelet counts displayed variability across different studies, generally trending higher than the normal range. Furthermore, elevated levels of C-reactive protein were noted in 13.6% of cases, while increased procalcitonin levels were observed in 10.6% of cases.24
Our meticulous review of clinical data on pediatric COVID-19 cases indicates a prevailing trend of mild to moderate disease severity, with varying proportions of asymptomatic patients across studies. While the majority of cases exhibit subtle clinical features, exceptional instances, such as a 1-year-old boy presenting with critical symptoms, underscore the need for heightened vigilance in recognizing atypical manifestations promptly, especially in pediatric cases where challenges in diagnosis may arise as given in Figure 3.
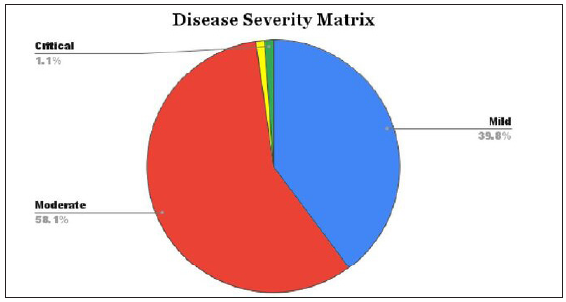
- Distribution of severity categories in cases of COVID-19 infection.
DISCUSSION
This systematic investigation meticulously collected and amalgamated prevailing observational studies concerning COVID-19 in the pediatric cohort. The surge in global COVID-19 cases is evident, with the most extensive epidemiological survey revealing a predominant demographic impact within the age range of 30–79 years, encompassing 87% of reported cases. It is crucial to highlight that advanced age and the presence of pre-existing medical conditions correlate with an elevated susceptibility to the deleterious effects of the virus. Conversely, the prevalence among the pediatric population remains considerably modest, constituting a mere 2.2% of the 44,672 confirmed cases, thereby yielding a conspicuously low crude mortality rate of 0.1%. This stands in sharp contradistinction to the elevated mortality rates documented in contemporary published data for the adult demographic, ranging from 2.3% to 14.6%.25,26
The study maintains adherence to standardized categorization, harmonizing pooled data accordingly. The results reveal a tendency towards mild to moderate disease severity among children, with mild cases often characterized by subtle and transient clinical features, contributing to varying proportions of asymptomatic patients across distinct studies. It’s worth noting that these outcomes may, in part, reflect the early stages of the COVID-19 outbreak, akin to observations in adults, where more symptomatic or severe cases were prevalent. This variability introduces a moderate level of heterogeneity in the pooled data concerning clinical features.
A male infant, aged one year, was categorized as critically ill. His presentation initially involved vomiting and diarrhea over a 6-days period, devoid of apparent cough or respiratory symptoms. However, his condition swiftly deteriorated post-admission, leading to shock and subsequent progression to acute respiratory distress syndrome (ARDS), necessitating mechanical ventilation.27 Furthermore, the patient exhibited nephropathy necessitating hemodialysis during the course of hospitalization. Recognizing such atypical presentations promptly poses heightened challenges in pediatric cases.
In comparison to a closely related virus, SARS-CoV, it’s evident that SARS-CoV-2 leads to milder disease. While the precise factors contributing to this phenomenon are still being investigated, some of these parameters are widely acknowledged. Numerous factors may underlie this phenomenon, with a crucial determinant in the attenuated course of COVID-19 in children being the lack of baseline inflammation. In contradistinction to adults, pediatric individuals exhibit a more robust immune response and demonstrate diminished vulnerability to pre-existing medical conditions.28 Severe respiratory complications requiring intensive care are infrequent among children in comparison to adults.29 Notably, children with pneumonia often encounter coinfections involving both viruses and bacteria, potentially enhancing their immune memory against a broader spectrum of pathogens. However, the applicability of this observation to pneumonia related to SARS-CoV-2 remains uncertain.
Another significant aspect is the emergence of multisystem inflammatory syndrome in children (MIS-C), temporally associated with COVID-19. MIS-C represents a recently recognized, infrequent, and potentially life-threatening hyperinflammatory condition characterized by features that overlap with both typical and incomplete presentations of Kawasaki disease and toxic shock syndrome.30-32 Moreover, comprehending the diverse risks associated with children can be intricate. Those in whom coronaviruses are identified in the respiratory tract may encounter viral coinfections in as much as two-thirds of the cases.33
An additional hypothesis warranting investigation regarding the reduced severity risk of COVID-19 in children compared to adults revolves around the attenuated expression of ACE2 in the nasal epithelia of children.34,35 The binding affinity of SARS-CoV-2 to ACE2 provides partial insight into why it induces less severe disease than SARS-CoV while retaining a high level of infectiousness. However, it is crucial to acknowledge that this explanation does not comprehensively address why children exhibit lower susceptibility to severe COVID-19. Previous studies have indicated that SARS-CoV induces higher ACE2 shedding than human coronavirus NL63.
The divergent regulation of ACE2 receptors holds close relevance to lung injury.36 Research indicates that aging induces changes in the pulmonary renin-angiotensin system, correlating with heightened inflammation and more pronounced lung injury in a rat model.37 Notably, smoking is a predominant factor among adults as opposed to children. An underlying hypothesis posits that smoking may elevate ACE2 expression, potentially augmenting the entry of coronaviruses into pulmonary epithelial cells.38
CONCLUSION
In our systematic analysis, several inherent limitations merit consideration. The COVID-19 outbreak occurred over two years ago, leading to a substantial accumulation of detailed descriptions, posing challenges in data relevance and reliability due to potential variations in employed assessment tools. Continuous follow-up studies are imperative for assessing long-term outcomes and potential sequelae, while the prevalence of multiple viral infections akin to COVID-19 in the same environment adds complexity to diagnosis. Additionally, the treatment strategy for children warrants further examination. In conclusion, the pediatric population tends to exhibit a less severe response to viral infections, with explanations subject to evolution as new patient cohorts are examined. The increased prevalence of asymptomatic and mild cases adds complexity to the diagnostic process and infection management.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Risk for transportation of coronavirus disease from Wuhan to other cities in China. Emerg Infect Dis. 2020;26:1049-52.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol. 2021;22:25-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Covid-19 vaccine-induced thrombosis and thrombocytopenia: First confirmed case from India. Indian J Hematol Blood Transfus. 2021;38:196-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antibodies against platelet factor 4 and their associated pathologies: from HIT/HITT to spontaneous HIT-like syndrome, to COVID-19, to VITT/TTS. Antibodies. 2022;11:7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Infants born to mothers with a new Coronavirus (COVID-19) Front Pediatr. 2020;8:104.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364-74.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prediction of the COVID-19 epidemic trends based on SEIR and AI models. PLoS One. 2021;16:e0245101.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-52.
- [CrossRef] [PubMed] [Google Scholar]
- Improved clinical symptoms and mortality among patients with severe or critical COVID-19 after convalescent plasma transfusion. Blood. 2020;136:755-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20:e276-e288.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical presentations and CT features of imported coronavirus disease 2019. Chin J Med Imaging Technol 2020:1-4.
- [Google Scholar]
- Coronavirus infections in children including COVID-19: An overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children: An overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39:355-68.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sequencing identifies multiple early introductions of SARS-CoV-2 to the New York City region. Genome Res. 2020;30:1781-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diagnosis, treatment, and prevention of 2019 novel Coronavirus infection in children: Experts’ consensus statement. World J Pediatr. 2020;16:223-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51-60.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Screening and severity of Coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. 2020;174:1009.
- [CrossRef] [PubMed] [Google Scholar]
- Children with covid-19 in pediatric emergency departments in Italy. N Engl J Med. 2020;383:187-90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Neurologic manifestations in an infant with COVID-19. Neurology. 2020;94:1100-2.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702.
- [CrossRef] [PubMed] [Google Scholar]
- Laboratory abnormalities in children with novel Coronavirus disease 2019. Clin Chem Lab Med. 2020;58:1135-8.
- [CrossRef] [PubMed] [Google Scholar]
- The profile of peripheral blood lymphocyte subsets and serum cytokines in children with 2019 novel Coronavirus pneumonia. J Infect. 2020;81:115-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical features of severe pediatric patients with Coronavirus disease 2019 in Wuhan: A single center’s observational study. World J Pediatr. 2020;16:251-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the chinese center for disease control and prevention. JAMA.. 2020;323:1239-42.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507-13.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical features of patients infected with 2019 novel Coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- First case of severe childhood novel coronavirus pneumonia in China. Zhonghua Er Ke Za Zhi. 2020;58:179-82.
- [CrossRef] [PubMed] [Google Scholar]
- Lessons from COVID-19 in children: Key hypotheses to guide preventative and therapeutic strategies. Clin Infect Dis. 2020;71:2006-13.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;34
- [CrossRef] [PubMed] [Google Scholar]
- Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Kawasaki-like disease: Emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741-3.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- COVID-19 and Kawasaki disease: Novel virus and novel case. Hosp Pediatr. 2020;10:537-40.
- [CrossRef] [PubMed] [Google Scholar]
- Human Coronavirus in hospitalized children with respiratory tract infections: A 9-year population-based study from Norway. J Infect Dis. 2019;219:1198-206.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84:1198-205.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Age-dependent changes in the pulmonary renin-angiotensin system are associated with severity of lung injury in a model of acute lung injury in rats. Crit Care Med. 2016;44:e1226-e1235.
- [CrossRef] [PubMed] [Google Scholar]
- Alternative roles of STAT3 and MAPK signaling pathways in the MMPs activation and progression of lung injury induced by cigarette smoke exposure in ACE2 knockout mice. Int J Biol Sci. 2016;12:454-65.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





