Translate this page into:
Comparison of hematologic parameters as predictors of severity in COVID-19: A single-center cross-sectional study
* Corresponding author: Dr Ruchi Kapoor, Department of Anaesthesiology, University College of Medical Sciences, Delhi, India. Email: rudoc@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gogoi P, Diwaker P, Kapoor R, Narang S, Goswami SJ. Comparison of hematologic parameters as predictors of severity in COVID-19: A single-center cross-sectional study. Ann Natl Acad Med Sci (India). 2024;60:164–7. doi: 10.25259/ANAMS-2023-6-9-(950)
Abstract
COVID-19 disease was a global pandemic that marred humanity globally from 2019 end till early 2023. The diseases caused by the novel SARS cov2 virus had a wide array of presentations, from asymptomatic to mild illness to severe acute respiratory illness, with patients presenting with ‘happy hypoxia and sudden deterioration of clinical condition to multiorgan involvement and proven to be fatal in many cases. There was an effort to identify a point-of-care test to screen patients so as to predict the disease course; therefore, this study was designed to compare hematologic parameters to predict the severity of the disease. This study showed that TLC,dNLR, and NLR can be effectively used in diagnosing severe COVID-19.
Keywords
COVID-19
haematologic parameters
severity
INTRODUCTION
The emergence of SARS-CoV-2, widely recognized as COVID-19, originated in December 2019 in Wuhan, China. Within no time, it evolved into a highly contagious pandemic, and its rapid spread and severity led to its classification as a global health emergency.1 SARS-CoV-2 belongs to the novel coronavirus family, Coronaviridae (subfamily Coronavirinae), with a host range spanning from bats to humans.2 COVID-19 is a complex ailment affecting multiple systems, but it primarily impacts the respiratory system.3 Individuals with mild-to moderate cases often exhibit flu-like symptoms—fever, dry cough, and breathlessness. Many recover through isolation and medical care, yet a subset experiences rapid deterioration or enters a critical state early on, indicating severe disease. This severe manifestation often results from an immune response imbalance leading to an excessive release of cytokines, termed the “cytokine storm” syndrome. Elevated cytokine levels trigger swift leukocyte recruitment to organs, particularly the lungs, culminating in acute respiratory distress syndrome (ARDS). The progression involves monocytes, macrophages, dendritic cells, and lymphocytes, collectively contributing to the cytokine storm. Critically ill patients deteriorate into nonresponsive respiratory failure, ARDS, and multiple organ dysfunction syndrome (MODS), posing significant challenges for medical professionals.
Prompt identification of high-risk patients and early intervention using point-of-care tests can facilitate targeted allocation of resources and categorization into mild, moderate, and severe groups. This strategic approach optimizes healthcare delivery, potentially leading to reduced mortality rates through specialized critical care. In addition, this approach aids in recognizing potential severity within mild to moderate cases, enabling intensified monitoring and timely intervention.
Essential to this approach are readily accessible biomarkers predicting systemic inflammation, potentially serving as prognostic indicators. Parameters like peripheral white blood cell (WBC) count, neutrophil-to-lymphocyte ratio (NLR), derived neutrophil-to-lymphocyte ratio (d-NLR, calculated by dividing neutrophil count by the result of WBC count minus neutrophil count), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) have established roles in predicting systemic inflammation across diverse diseases. Elevated NLR correlates with COVID-19 severity and mortality.4 Though studies have explored NLR, d-NLR, PLR, and LMR as prognostic indicators for COVID-19, a consensus on their relationship with clinical prognosis remains elusive. Consequently, our study aims to evaluate these hematological parameters in both mild to moderate and severe COVID-19 cases at our center. This study seeks to compare complete blood count (CBC) findings, NLR, d-NLR, PLR, and LMR between mild to moderate and severe COVID-19 cases.
MATERIAL AND METHODS
This cross-sectional study received ethical approval from the Institutional Ethical Committee for Human Research. The study involved a sample of 27 COVID-19 cases who were admitted to the center. Among these, 12 cases were categorized as mild-to-moderate COVID-19, and 15 cases fell under the severe category. The inclusion criteria included admitted patients with confirmed SARS-CoV-2 virus infection, verified by a positive reverse transcriptase polymerase chain reaction (RT-PCR) test. Both mild-to-moderate and severe cases were considered for inclusion, while pediatric COVID-19 cases were excluded from the study.
The categorization of cases into mild, moderate, and severe was based on the clinical criteria established by the Ministry of Health and Family Welfare (MOHFW), Government of India (Clinical Management Protocol COVID-19 Version 6). Mild COVID-19 cases exhibited symptoms of upper respiratory tract infection without shortness of breath and with normal SpO2 levels. Moderate cases showed signs of difficulty in breathing, fever, cough, SPO2 levels between 90 and 93% on room air, and a respiratory rate of ≥24/min. Severe cases displayed clinical pneumonia symptoms with SPO2 levels below 90% on room air, a respiratory rate exceeding 30/min, and indications of ARDS, sepsis, or septic shock.
Each case underwent detailed clinical history and examination recording. Venous blood (2 mL) was collected and stored in Ethylene diamine tetra acetic acid (EDTA) vials. Using an Automated Hematology Analyzer (Mindray BC 6800), various CBCs (including hemoglobin, total red blood cell (RBC) count, RBC indices, total leukocyte count (TLC), differential leukocyte count, and platelet count) were measured. NLR, dNLR, PLR, and LMR were manually calculated from CBC values. dNLR was computed as the absolute neutrophil count divided by the difference between the total leukocyte count and absolute neutrophil count.
Statistical analysis was conducted using SPSS v.26. For comparison between the two patient categories, the independent t-test was employed when data exhibited normal distribution and equal variances. In cases of skewed and kurtotic data, the Mann–Whitney U test was utilized. The receiver operator characteristic (ROC) curve, along with Youden J statistics, were employed to identify cutoff values. In addition, binary logistic regression analysis was performed to assess variables as prognostic factors, determining odds ratios. A significance level of p < 0.05 was considered statistically significant.
RESULTS
Table 1 presents an overview of the collected data. Our study found statistically significant differences in several parameters between severe and mild COVID-19 patients. Specifically, the TLC exhibited a significant increase (p < 0.05) in severe COVID-19 cases (Mean ± SD: 19840 ± 8305) compared to mild cases (Mean ± SD: 7508 ± 2227). NLR and dNLR also showed substantial elevation (p < 0.05) in severe cases (NLR Mean ± SD: 11.2 ± 6.8, dNLRMean ± SD: 6.7 ± 3.3) in contrast to mild cases (NLR Mean ± SD: 5.4 ± 4.2, dNLRMean ± SD: 3.5 ± 2.7). However, no statistically significant difference was observed between the two groups for the remaining parameters, including platelet count, PLR, and LMR.
| Parameter | Senstivity | Specificity |
|---|---|---|
| TLC | 93.3% | 91.7% |
| NLR | 73.3% | 72.7% |
| dNLR | 80% | 81.8% |
TLC: Total leukocyte count, NLR: Neutrophil-to-lymphocyte ratio, d-NLR: derived neutrophil-to-lymphocyte ratio.
To assess the diagnostic utility of these parameters, an ROC curve analysis was conducted, yielding the corresponding area under the curve (AUC) values. Figure 1 shows the ROC curve which has been attached as a jpg attachement highlighted by the icon. For the total leukocyte count (TLC), the AUC was 0.976. The AUCs for NLR and dNLR were 0.770 and 0.800, respectively. Utilizing Youden J statistics (Sensitivity + Specificity − 1), optimal cutoff values were identified: 10,950 for TLC, 6.19 for NLR, and 4.13 for dNLR. These values were then evaluated against the clinical diagnosis as the gold standard. As a result, the total leukocyte count (TLC) demonstrated a sensitivity of 93.3% and specificity of 91.7%; neutrophil-to-lymphocyte ratio (NLR) exhibited a sensitivity of 73.3% and specificity of 72.7%; dNLR showed a sensitivity of 80% and specificity of 81.8% in identifying severe COVID-19 infections.
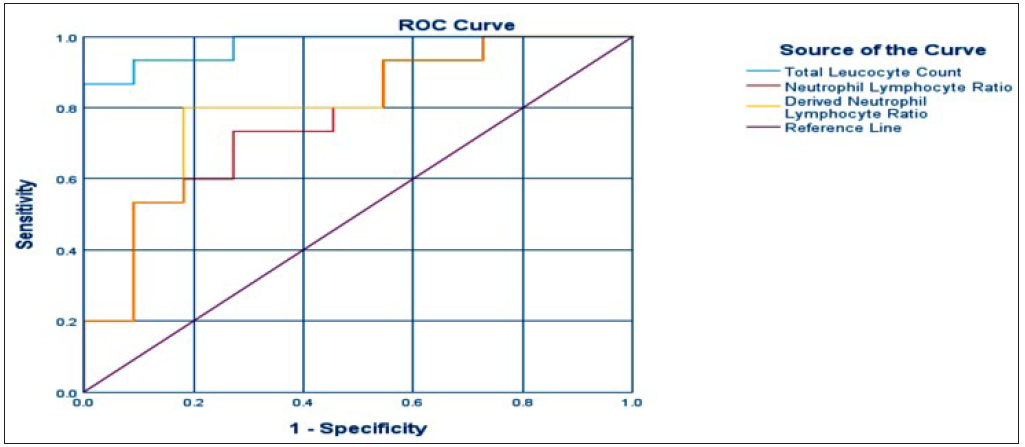
- ROC: Receiver operator characteristic curve.
Furthermore, a binary logistic regression analysis was performed to ascertain the prognostic significance of TLC, NLR, and dNLR for severe disease and unfavorable clinical outcomes. The calculated odds ratio was 1.001 for TLC (P < 0.05), 1.463 for dNLR (p < 0.05), and 1.221 for NLR (p < 0.05). These findings underscore the potential of these parameters as indicators of poor prognosis in severe COVID-19 cases.
DISCUSSION
The phenomenon of the cytokine storm, an acute hyperinflammatory response, has been implicated in the pathogenesis of severe illnesses associated with viral infections, cancer, sepsis, and similar conditions. This immune system dysregulation is also believed to contribute to the severity of COVID-19, caused by the novel coronavirus SARS-CoV-2.5 This phenomenon underlies several severe manifestations of COVID-19, including ARDS, thromboembolic events, acute kidney injury (AKI), and vasculitis. Studies have consistently demonstrated elevated levels of various inflammatory markers such as granulocyte-macrophage colony stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP1), macrophage inflammatory protein 1 alpha (MIP1A), tumor necrosis protein alpha (TNF), interleukin 6, C-reactive protein, ferritin, and procalcitonin in severe COVID-19 cases, suggesting a state of hyperinflammation.5
Our study revealed a significant elevation in TLC values among severe COVID-19 patients compared with mild cases. This aligns with previous research indicating that severe COVID-19 infection is associated with an increased neutrophil count and decreased lymphocyte count.6 We also observed significantly higher NLR and dNLR values (p < 0.05) in severe cases, reflective of the prevailing imbalance between elevated neutrophil and diminished lymphocyte counts—a hallmark of COVID-19 infection. The exaggerated immune response in severe COVID-19 triggers the release of cytokines such as GM-CSF, interleukin 6, interleukin 8, and tumor necrosis factor alpha, contributing to heightened neutrophil production. Concurrently, secondary bacterial infections can also lead to neutrophilia, while lymphopenia may arise from cytokine-induced inhibition, increased apoptosis, and lymphocyte redistribution within the lymphatic system. These observations are in concurrence with previous studies.
Our study demonstrated that TLC, NLR, and dNLR possess significant clinical relevance in diagnosing severe COVID-19 infections. ROC curve analysis highlighted that TLC exhibited the largest AUC at 0.976, followed by dNLR with AUC 0.800, and NLR with AUC 0.770. This indicates that, according to our findings, TLC holds the most diagnostic value, followed by dNLR, which surpassed NLR in diagnosing severe COVID-19 infections. This contrasts with some previous studies that emphasized NLR’s higher sensitivity and specificity than dNLR.7 Through Youden’s J statistic, optimal cutoff values were determined: 10,950 for TLC, 6.19 for NLR, and 4.13 for dNLR. By applying these cutoff values, TLC emerged as the most sensitive and specific indicator, followed by dNLR, for identifying severe COVID-19 cases. The cutoff value for NLR in our study resonates with that in the study by Prozan et al.,8 albeit slightly deviating from a few other studies. Binary logistic regression analysis further corroborated these findings, revealing statistically significant results and reinforcing the variables as indicators of poor prognostic factors for severe disease, consistent with prior research. While we anticipated differences in platelet count, PLR, and LMR between the two groups based on previous studies, our analysis did not yield statistically significant differences. A possible explanation for this observation could be the limited sample size in our study.
CONCLUSION
Severe complications stemming from COVID-19 demand immediate attention due to their potential life-threatening nature. Identifying poor prognostic factors early on and intervening promptly can yield significant benefits. Our study underscores the effective utilization of easily accessible circulatory biomarkers—TLC, dNLR, and NLR—in diagnosing severe COVID-19 infections. These biomarkers indirectly reflect the hyperinflammatory response associated with severe disease. With vigilant monitoring, we stand to identify high-risk individuals promptly, enabling timely interventions that can reduce mortality rates
Ethical approval
The research/study approved by the Guru Teg Bahadur Hospital Ethics Committee, GTBHEC Protocol number - GTBHEC2021/P-141, dated 12-04-2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- The novel coronavirus originating in Wuhan, China: Challenges for Global Health Governance. JAMA. 2020;323:709-10.
- [CrossRef] [PubMed] [Google Scholar]
- Coronavirus disease 2019 (COVID-19): Current status and future perspectives. Int J Antimicrob Agents. 2020;55:105951.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- COVID-19: A complex multisystem disorder. Br J Anaesth. 2020;125:238-42.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Neutrophil-to-Lymphocyte Ratio (NLR) is a promising predictor of mortality and admission to intensive care unit of COVID-19 patients. J Clin Med. 2022;11:2235.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cytokine storm: The primary determinant for the pathophysiological evolution of COVID-19 deterioration. Front Immunol. 2021;12:589095.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in. Lancet. 2020;395:497-506.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prognostic value of neutrophil-to-lymphocyte ratio in COVID-19 compared with Influenza and respiratory syncytial virus infection. Scientific reports. 2021;11:21519.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





