Translate this page into:
Evaluation of repeat colostomy biopsy in Hirschsprung’s disease patients with absent ganglion cells at the colostomy site
* Corresponding author: Dr. Anand Pandey, Department of Pediatric Surgery, King George’s Medical University, Lucknow, Uttar Pradesh, India. dranand27@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Tyagi N, Pandey A, Gupta SK, Pant N, Varma M, Rawat J. Evaluation of repeat colostomy biopsy in Hirschsprung’s disease patients with absent ganglion cells at the colostomy site. Ann Natl Acad Med Sci (India). doi: 10.25259/ANAMS_8_2024
Abstract
Objectives
Hirschsprung’s disease (HD) is characterized by the absence of ganglion cells in the myenteric and submucosal plexuses of the intestine. Many Indian centers prefer a two-stage procedure. In the first stage, biopsies are taken from the peritoneal reflection, transition zone (TZ), and colostomy site. There are instances of ganglion cells being absent at the colostomy site after the first surgery. This paper deals with the management of such cases.
Material and Methods
It was a retrospective observational study from April 2015 to 2023. All patients with HD confirmed by histopathological examination (HPE) based on hematoxylin and eosin (HE) staining and not having ganglion cells at the colostomy site were included.
Results
During the study period, there were 311 patients. Of these, 25 (8%) had the first report of absent ganglion cells at the affected and colostomy site. The mean age of the patients was 2.16 years. Of these, two patients had abdominal distension, which were managed by colostomy dilation and laxatives. The remaining patients had no such issues. Repeat HPE of the 23 asymptomatic patients showed the presence of ganglion cells. The remaining two patients underwent revision colostomy.
Conclusion
Repeat colostomy site biopsy in HD patients with absent ganglion cells at TZ and colostomy sites may be beneficial in places where facilities for frozen section and acetylcholinesterase staining are limited. We can also avoid laparotomies for multiple colonic biopsies.
Keywords
Biopsy
Colostomy
Ganglion cells
Hirschsprung’s disease
INTRODUCTION
Hirschsprung’s disease (HD) is characterized by the absence of ganglion cells in the myenteric and submucosal plexuses of the intestine. It is also known as a congenital megacolon.1 After clinical suspicion, the diagnosis is confirmed by water-soluble contrast enema and histopathology examination (HPE).1 The contrast enema shows a pathognomonic finding of HD, which is a transition zone (TZ), between the normal and aganglionic bowel [Figure 1]. The HPE shows an absence of ganglion cells in the myenteric and submucosal plexuses.
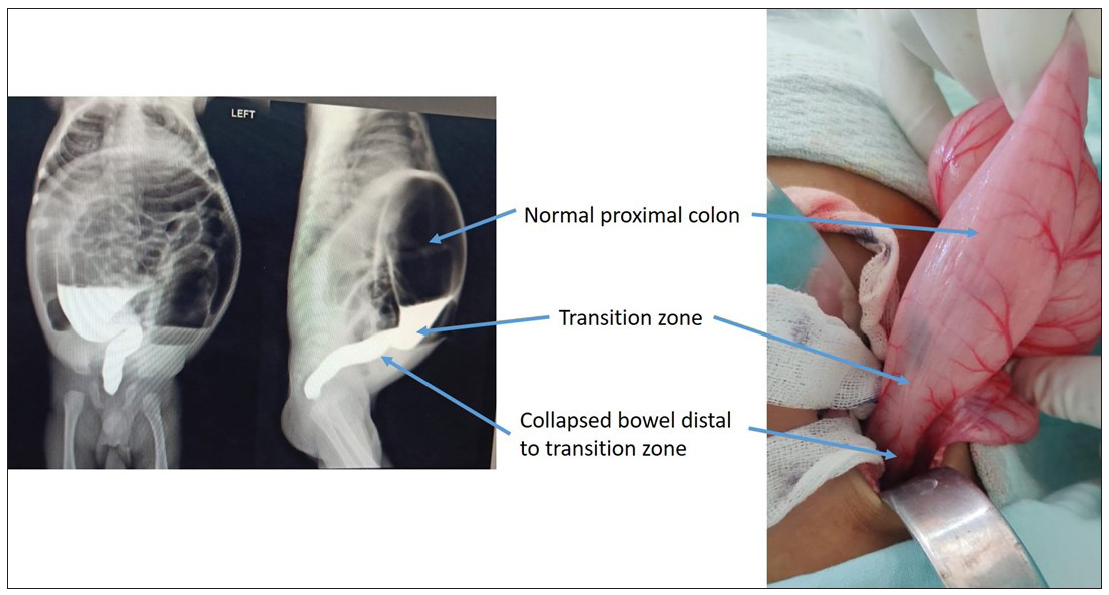
- Intraoperative correlation of water-soluble contrast enema. The figure shows the transition zone between the normal and distal aganglionic colon. The colostomy is made in the normal colon about 10 cm from the transition zone. The biopsy is obtained from the colostomy site, transition zone, and colon distal to the transition zone.
Currently, single-stage management is preferred in many Western centers.2 However, many Indian centers, including ours, prefer a two-stage procedure. Stage 1 is a loop colostomy at least 5 cm proximal to the transition zone, followed by definitive surgery (Duhamel or other procedures).3 The second stage involves mobilizing the colostomy mobilized and pulling the bowel down for definitive repair.
During the first stage of surgery, tissue samples are obtained at the peritoneal reflection of the rectum, TZ, and colostomy site for histopathological confirmation. Scanty or absent ganglion cells at the TZ and absent ganglion cells beyond the TZ confirm the histopathological diagnosis of HD, and the presence of ganglion cells at the colostomy sites ensures that the pulled-through segment in the second stage will be normal.3
There are instances of the absence of ganglion cells at the colostomy site. Definitive surgery is not safe if the status of ganglion cells is uncertain. This paper deals with the management of such cases.
MATERIAL AND METHODS
It was a retrospective observational study conducted in the Department of Pediatric Surgery, King George’s Medical University Hospital, from April 2015 to April 2023. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines endorsed by the EQUATOR Network for conducting this study [Supplementary Material].
All patients with no stool passage in the first 24-48 hours and a clinical suspicion of HD underwent a water-soluble contrast enema to confirm the diagnosis. If a TZ was noted in the dye study, a laparotomy was performed under general anesthesia. A loop colostomy was made about 10 cm from the TZ, which was noted intraoperatively, and a biopsy was taken from the colostomy site, TZ, and rectosigmoid region. It included approximately 0.5 cm of seromuscular tissue from the anterior surface of the bowel. The distal bowel was not resected at that time.
All patients with confirmed HD, diagnosed by HPE based on hematoxylin and eosin (HE) staining, and the absence of ganglion cells at the colostomy site were included. All other patients were excluded from the study. The management protocol was followed as per Figure 2. All patients were assessed for the age of presentation, sex, status of stool discharge from the stoma site, and history of abdominal distension at the first HPE report.
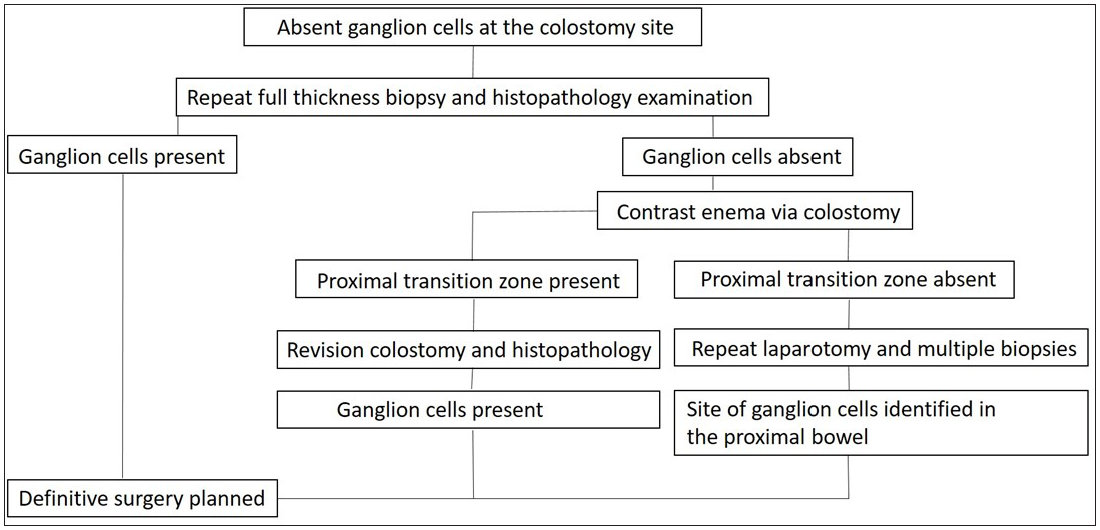
- Management algorithm for patients diagnosed with Hirschsprung’s disease and absent ganglion cells at the colostomy site.
Procedure for obtaining a colostomy site biopsy
After confirming anesthetic fitness, a daycare procedure under short general anesthesia was performed. A full-thickness piece was obtained from one side of the colostomy. It is like a wedge from a circle. The wedge was at least 0.5 to 1 cm wide at the base. It was immediately placed in 10% neutral buffered formalin and sent to the Pathology Department. After securing hemostasis, the created defect was closed with a 3-0 or 4-0 absorbable braided suture. The patients were discharged on the same day.
RESULTS
During the study period of eight years, there were 311 patients diagnosed with HD based on the HPE reports. Of these, 25 (8%) had absent ganglion cells in the rectosigmoid region, TZ, and colostomy site for the first time [Table 1].
| S. No | Age | Sex | Passing stool via colsotomy | Abdominal distension | Ganglion cells in repeat biopsy | Dye study done |
|---|---|---|---|---|---|---|
| 1 | 4 yr | m | no | Intermittent | absent | Proximal TZ seen |
| 2 | 1 yr | m | yes | no | cells present | no |
| 3 | 1 yr | m | yes | no | cells present | no |
| 4 | 2yr | m | yes | no | cells present | no |
| 5 | 1 yr | m | yes | no | cells present | no |
| 6 | 2 yr | m | yes | no | cells present | no |
| 7 | 1 yr | m | yes | no | cells present | no |
| 8 | 8 m | m | yes | no | cells present | no |
| 9 | 2 m | m | yes | no | cells present | no |
| 10 | 6 m | m | yes | no | cells present | no |
| 11 | 4 yr | m | yes | no | cells present | no |
| 12 | 4 yr | m | yes | no | cells present | no |
| 13 | 4 m | m | yes | no | cells present | no |
| 14 | 4 m | m | yes | no | cells present | no |
| 15 | 7 m | f | yes | no | cells present | no |
| 16 | 7 m | f | yes | no | cells present | no |
| 17 | 5 yr | f | yes | no | cells present | no |
| 18 | 7 yr | m | yes | no | cells present | no |
| 19 | 4 yr | m | yes | no | cells present | no |
| 20 | 6 yr | f | yes | no | cells present | no |
| 21 | 5 yr | m | yes | no | cells present | no |
| 22 | 1 yr | m | yes | no | cells present | no |
| 23 | 1 yr | m | yes | no | cells present | no |
| 24 | 1.5 yr | m | yes | no | cells present | no |
| 25 | 7m | m | no | Mild | absent | Proximal TZ seen |
Yr: Years, m: Months, TZ: Transitional zone
At the time of the first presentation, all patients complained of abdominal distension and the inability to pass meconium. There was no patient with long-segment HD in this study. The mean age of the patients was 2.16 years (range- 2 months to 7 years). The male-to-female ratio was about 4:1. After the colostomy (first stage of surgery), abdominal distension resolved in 23 patients, and they were passing stools normally from the colostomy site. Two patients still suffered from abdominal distension and were able to pass stools with the help of colostomy dilation and laxatives. A dye study via colostomy revealed a proximal TZ [Figure 3].
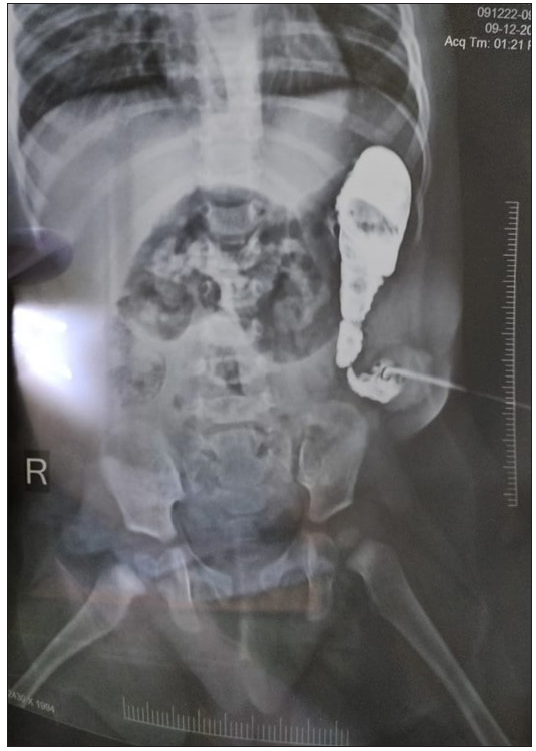
- Proximal colostogram done in a patient with a complaint of constipation after colostomy was made. A transition zone is visible in the proximal colon.
The repeat HPE obtained from the colostomy site of the 23 asymptomatic patients showed the presence of ganglion cells, while ganglion cells could not be seen in the remaining two patients. These two patients underwent revision colostomy, about 10 cm from the previous colostomy. Their fresh report showed the presence of ganglion cells at the new colostomy site. Both of them are currently waiting for definitive surgery. Of the 23 patients with normal reports of repeat HPE, 19 have undergone a definitive procedure (the Duhamel operation). They had no problems during the follow-up.
DISCUSSION
The patients were referred elsewhere for frozen section biopsy before the colostomy biopsy was ideated. This led to a loss of follow-up. Besides, many patients did not go to the referred hospitals. This was due to financial issues, transportation issues, lack of interest in the needs of the baby, etc.
Out of the 25 patients, most (23) had no clinical evidence of a non-functioning colostomy. It suggests that the bowel was functioning normally, and it is indirect evidence of the presence of ganglion cells. However, both medicolegally and ethically, it is not advisable to proceed with definitive surgery without evidence of the presence of the ganglion cells on HPE.
Various causes have been suggested for the non-visualization of the ganglion cells. These include inadequate quantity, disorientation of the tissue sent for HPE, limited exposure of the pathologist to this entity, and normal immature ganglion cells in newborns.4 Crushing artifacts may also result if the tissue sample is handled too hard or if the submucosa is grasped while trying to get a seromuscular biopsy.5
It can be reasonably argued that a frozen section biopsy can provide a rapid diagnosis,6 and a colostomy biopsy is not needed. However, it is better suited for centers with good availability of biopsy facilities as well as manpower. If a center does not have these facilities, a frozen section biopsy is out of the question. Besides, the frozen section is not always reliable.7 Acetylcholinesterase staining has made the diagnosis of HD easier;8 however, our center, like most others in India, does not have this facility.
It is important to consider the instance of the uncertainty of a TZ, especially in neonates. This becomes tricky when the frozen section facility is not available, like in our set up. It is then better to incease the incision and rule out long-segment HD. Since TZ can extend up to 13 cm in long-segment HD,9 a minimum 5 cm beyond the probable TZ may avoid the possibility of making a colostomy in the aganglionic colon.
While performing the literature review, we could not retrieve any articles on such cases. It could be that due to the availability of frozen section biopsy equipment and acetylcholinesterase staining, such a situation has not been encountered. Another possibility is that a repeat laparotomy and multiple biopsies were performed on encountering such a situation. However, it is speculation, and we do not have evidence for this statement.
From the flowchart [Figure 2], the rationale for performing a dye study at the colostomy site may be judged. Through the dye study, we can make a preoperative decision about the TZ and a future stoma site; otherwise, we would be taking multiple biopsies and a colostomy site would remain undecided. The limitation of this study is its retrospective nature; however, all similar studies can have missing data. We believe that the present data is sufficient to claim the benefit of the procedure used.
CONCLUSION
Repeat colostomy site biopsy in HD patients with absent ganglion cells at TZ and colostomy sites may be beneficial in places where facilities for frozen section and acetylcholinesterase staining are limited. We can also avoid laparotomies for multiple colonic biopsies.
Authors’ contributions
AP: Conceptualized the idea; NT, SKG, and AP: Performed the literature review and collected the data; AP, NT, and MV: Wrote the first draft of the manuscript. JDR and NP: Reviewed the manuscript; AP and JDR: Prepared the final draft. The final version was read and approved by all authors.
Ethical approval
Institutional Review Board approval is not required as it is a retrospective study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Hirschsprung disease. In: Holcomb GW III, Murphy JP, St.Peter SD, eds. Holcomb and Ashcraft’s pediatric surgery (7th edition). Philadelphia PA: Elsevier; 2020. p. :557-76.
- [Google Scholar]
- The role of stomas in the initial and long-term management of Hirschsprung disease. J Pediatr Surg. 2023;58:236-40.
- [CrossRef] [PubMed] [Google Scholar]
- Modified Duhamel’s two-staged procedure for hirschsprung’s disease: further modifications for improved outcomes. J Indian Assoc Pediatr Surg. 2020;25:269-75.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hirschsprung disease. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
- [Google Scholar]
- Gastrointestinal neuromuscular pathology: guidelines for histological techniques and reporting on behalf of the Gastro 2009 International Working Group. Acta Neuropathol. 2009;118:271-301.
- [CrossRef] [PubMed] [Google Scholar]
- Surgery, Surgical pathology, and postoperative management of patients with hirschsprung disease. Pediatr Dev Pathol. 2020;23:23-39.
- [CrossRef] [PubMed] [Google Scholar]
- Can we rely on frozen sections of a rectal biopsy for one-stage trans-anal pull-through operation in Hirschsprung’s disease? Iran J Pediatr. 2011;21:72-6.
- [PubMed] [PubMed Central] [Google Scholar]
- Diagnosis and differential diagnosis of Hirschsprung’s disease. In: Holschneider AM, Puri P, eds. Hirschsprung’s disease and allied disorders (3rd edition). Berlin Heidelberg: Springer-Verlag; 2008. p. :185-98.
- [Google Scholar]
- The extent of the transition zone in hirschsprung disease. J Pediatr Sur.. 2019;54:2318-24.
- [Google Scholar]






