Translate this page into:
HCV Seroreactivity and Detection of HCV RNA in Hepatitis Cases
Correspondence: Dr. Mahantesh B. Nagamoti, Professor of Microbiology, J.N. Medical College, KLE University, Belagavi, Karnataka. Email: drmbnagamoti@gmail.com
Abstract
Background:
Hepatitis C virus (HCV) known to be associated with wide variety of liver pathology. It is less studied in India as compared to western region.
Methods:
Suspected patients sera screened for HCV by ELISA and confirmed with reverse transcription polymerase chain reaction (RT-PCR) along with routine investigations and liver profile. All HCV positive patients were undergone liver biopsy.
Results:
All 24 HCV ELISA reactive and two ELISA indeterminate sera are confirmed by RT- PCR. The liver biopsy of these patients showed normal picture (19.2%), Acute hepatitis (11.5%), Chronic hepatitis (23.7%), Cirrhosis (34.72%), Hepato-cellular carcinoma (HCC) (15.38%). ALT levels were not significant.
Conclusion:
All the suspected HCV cases need to be confirmed for HCV by RT-PCR.
Keywords
Hepatitis C virus (HCV)
HCV RT-PCR
ALT levels
liver pathology.
Introduction
Hepatitis C virus (HCV) is a RNA virus, belongs to Hepacalci virus genus, transmitted parenterally leading to acute to chronic hepatitis and rarely may cause hepato-cellular carcinoma (1, 2). It is slowly progressive infection, affecting about 170 million people worldwide (3). There is significant geographic variation of prevalence of HCV across various regions of India (4). Approximately, 12-18 million people are infected with HCV in India having prevalence around 1 to 1.5% (5).
Due to rapidity and easiness, ELISA and rapid tests are most commonly used to detect HCV reactivity. Molecular detection methods are effective to detect the infection and also help to record changes in the period of therapy and to monitor the period of treatment. Even though, these techniques are more sensitive, rapid but require sophistication and have some limitations (6-8).
There is paucity in the epidemiology and clinical picture of HCV in India. There are very few reports from North Karnataka region. In this connection, we have screened the suspected hepatitis patients for HCV, using ELISA along with their liver pathology and further confirmed with reverse transcription polymerase chain reaction (RT-PCR) for HCV.
Materials and Methods
Subjects: A total of 612 sera from suspected cases of hepatitis were collected from the patients were are attending KLE Society's DR Prabhakar Kore Hospital & MRC Belagavi, (Karnataka) India, a teaching Hospital attached to the J N Medical College KLE University Belagavi, for testing of HCV, after taking approval by Institutional Ethical Committee. A detailed history and risk factors were noted and detailed investigations were carried out including liver profile (biochemistry/ biopsy).
Serology: All the sera were tested for HCV by ELISA using commercial kits (X-cyton India, Ranbaxy Diagnostics, England and Innogenetics Belgium) according to manufacturer's instructions. All HCV reactive and indeterminate sera were further confirmed by repeat ELISA.
RT-PCR: RT-PCR technique was used to detect HCV RNA, employing the primers from a highly conserved 5' Non Coding Region (5'NCR) (Table 1). Pre and post amplifications steps were performed in different sections, situated on two different floors of the Hepatitis Division of NIV, Pune. Positive and negative controls were included in every PCR experiment.
RNA Extraction: RNA was extracted from the patient's plasma samples, using Trizol L.S. reagent (Invitrogen). The extracted RNA was stored at 20°C until the PCR reaction was put up.
-
Reverse Transcription-PCR: Nested PCR was performed using 50 μ,L of the extracted RNA as template. Briefly, RT- PCR was carried out in 100 μ,L of reaction mixture containing 25 mM dNTPs, 50 ng/mL BSA, 10X PCR buffer, 20 pmol outer primers JENS1 and JENS 2, 0.5 mL of AMV reverse transcriptase (Promega) and 0.5 U of Taq DNA polymerase (Promega). The above mixture was incubated at 42°C for 1 hour. Amplification was performed for 35 cycles in an automated Thermocycler (Perkin Elmer Cetus), with denaturation at 94oC for 1 minute. Primer Annealing at 55oC for 1 min and amplification at 72oC for 1 min. Final extension was at 72oC for 2 min.
Five μ,L of the amplified product of the first PCR cycle was used for the second round of PCR (35 cycles) with inner primers. For 50 μ,L reaction master mix was prepared using Primer (Jens 3 and Jens 4), Taq polymerase, dNTPs and DW. Amplification was performed for 35 cycles in an automated Thermocycler (Perkin Elmer Cetus), with denaturation at 94oC for 1 minute. Primer Annealing at 55oC for 1 min and amplification at 72oC for 1 min. Final extension was at 72oC for 3 min.
Detection of the RT-PCR product : A 250 bp product was detected in an ethidium bromide stained 2% Agarose gel and compared with a molecular weight marker 100 bp ladder. Gel photograph was taken using Gene snap from Synergie US gel documentation system (Fig. 1).
| Primer | Sequence |
|---|---|
| Jens 1 | 51 ACT GTC TTC ACG CAG AAA GCG TCT AGC CAT ‘3’ |
| Jens 2 | 51 CGA GAC CTC CCG GGG CAC TCG CAA GCA CCC ‘3’ |
| Jens 3 | 5’ ACG CAG AAA GCG TCT AGC CA T GGC GTT AGT 3’ |
| Jens 4 | 5’ TCC CGG GGC ACT CGC AAG CAC CCT ATC AGG 3’ |
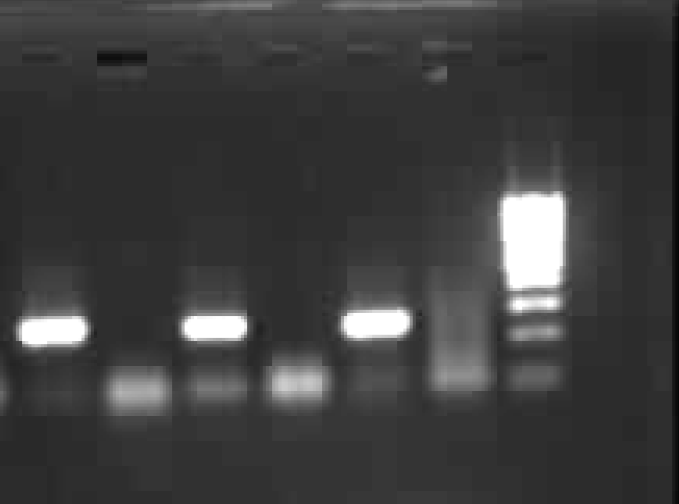
- HCV RNA - RT PCR results (lane 1, 3, 5 HCV positive sera, Lane 2,4 & 6 were negative sera and lane 7 Mol wt marker 100 bp ladder (left to right).
Results
Of the 612 suspected cases of hepatitis, 24 (3.92%) of the sera turns out to be HCV reactive by ELISA and two were indeterminate (0.32%). All HCV-ELISA reactive 24 cases and two indeterminate (0.32%) were confirmed by RT-PCR (4.24%). Of them 16 were males (66.66%) and 10 (41.67%) were female patients. The youngest was 20 year old and the oldest was 55 years. The mean age for the male patients was 38.44 years with a standard deviation of 9.99 and that for female patients was 36.63 years with a standard deviation 9.3. There was no significant variation between the mean ages of male and female patients (t= 0.29, df = 22, p = 0.78).
The ALT levels (Mean±SD) in the HCV infected patients were 73.87±50.73 (were statistically significant ‘p’ < 0.001). The ALT levels in all our patients with cirrhosis, acute hepatitis and in those with normal liver cell morphology was similar (58.5 IU). On the other hand, highest ALT levels were observed in patients with chronic hepatitis (114.17± 87.69). Moderately high ALT levels were found in patients with hepatocellular carcinoma.
The analysis of liver biopsy of these 26 patients were as follows; Normal-05 (19.2%), Acute hepatitis - 03 (11.5%), Chronic hepatitis - 06 (23.7%), Cirrhosis - 08 (34.72%), Hepato-cellular carcinoma (HCC) - 04 (15.38%).
Discussion
HCV infection known to be associated with wide variety of changes in the liver (9). HCV infection in our case also proved that it is associated with multiple histological changes including acute to chronic hepatitis and cirrhotic changes with HCC. Majority of the western studies proved the presence of HCV infection in cirrhotics in 65% of the cases and in India it is less (10-24%) (10). The association is slightly higher in our study as compare to Indian studies. It may be due to the fact that, the prevalence of HCV in chronic liver disease varies according to the background endemicity of HCV in the population (11).
We have observed, HCV viraemia in the presence of specific antibodies was observed in 24/62 (4.24%) and absence of specific antibodies was observed in two of our patients. The same has been observed by Marin et al (12). HCV RNA detection may be said to provide early diagnosis of HCV infection. The reason of HCV being negative by ELISA may be because of genetic heterogeneity or a weakened host immune response or due to molecular techniques, which are more sensitive than ELISA (9, 13-15).
It is a well known fact that, ALT has also been widely investigated as a surrogate marker for liver pathology and HCV infection (15). ALT is known to fluctuate in chronic HCV infection, and recent studies suggest that elevated levels do not correlate with viraemia (15). Herewith we also found that ALT levels are variable in various stages of the diseases. This finding suggests that, the serum ALT levels are not the reliable predictors of HCV - induced liver pathology.
We have observed a significant correlation between HCV reactivity with all types of histological lesions of the liver. Further this association may be well co-related, if we have titrated the HCV RNA using real time PCR. Herewith we conclude that, HCV RNA detection can be applied in all suspected hepatitis cases especially in early infective cases and ALT levels are insignificant in HCV liver pathology.
References
- Virus Taxonomy-Sixth report of the International committee on taxonomy of viruses. Vienna and New York: Springer- Verlag; 1995.
- [CrossRef] [Google Scholar]
- Hepatitis C antibody prevalence in blood donors in different governorates in Egypt. Trans R Soc Trop Med Hyg. 1997;91(3):271-274.
- [CrossRef] [PubMed] [Google Scholar]
- A study of changing trends of prevalence and genotypic distribution of hepatitis C virus among high risk groups in North India. Indian J Med Microbiol. 2013;31(4):354-359.
- [CrossRef] [PubMed] [Google Scholar]
- Future of therapy for Hepatitis C in India: a matter of accessibility and affordability? J Clin Exp Hepatol. 2014;4(2):85-86.
- [CrossRef] [PubMed] [Google Scholar]
- Importance of primer selection for the detection of hepatitis C virus RNA with the polymerase chain reaction assay. Proc Natl Acad Sci USA. 1992;89:187-191.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of the 5' noncoding region versus the NS5b region in genotyping hepatitis C virus isolates from blood donors in France. J Clin Microbiol. 2006;44:2051-2056.
- [CrossRef] [PubMed] [Google Scholar]
- A novel nested reverse-transcriptase polymerase chain reaction method for rapid hepatitis C virus detection and genotyping. Indian J Med Microbiol. 2014;32(2):130-136.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis of hepatitis C virus-associated liver disease in India: comparison of HCV antibody assay with a polymerase chain reaction for the 59 noncoding region. J Med Virol. 1994;44:176-179.
- [CrossRef] [PubMed] [Google Scholar]
- HCV sero-reactivity and detection of HCV RNA in cirrhotics. Diagn MicrobioI Infect Dis. 1999;35:209-213.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for the screening, care and treatment of persons with hepatitis C infection. Geneva: World Health Organization; 2014. Available from: http://apps.who.int/medicinedocs/documents/s22180en/s2218 0en.pdf
- [Google Scholar]
- Clinical significance of serum hepatitis C virus (HCV) RNA as marker of HCV infection. J Clin Microbiol. 1994;32:3008-3012.
- [CrossRef] [PubMed] [Google Scholar]
- Serological responses to different genotypes of hepatitis C virus in France. J Clin Microbiol. 1994;32:211-212.
- [CrossRef] [PubMed] [Google Scholar]
- A long term study of hepatitis C virus replication in non A, non B hepatitis. N Engl J Med. 1991;325:98-104.
- [CrossRef] [PubMed] [Google Scholar]
- Absence of extensive genetic heterogeneity of hepatitis C virus in antibody negative chronic hepatitis C. J Med Virol. 1996;49:87-90.
- [CrossRef] [Google Scholar]





