Translate this page into:
Metastatic disease in primary squamous cell carcinoma of ichthyosis uteri
* Corresponding author: Dr. Riddhi Jaiswal, MD, Department of Pathology, King Georges Medical University, Lucknow, India. riddhiadvay@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mathur P, Jaiswal R. Metastatic disease in primary squamous cell carcinoma of ichthyosis uteri. Ann Natl Acad Med Sci (India). doi: 10.25259/ANAMS_58_2024
Abstract
Ichthyosis uteri, is an exceedingly uncommon entity, in which the endometrial surface is completely replaced by a multilayered squamous epithelium. Literature reports this condition to be benign, with dysplasia, anaplasia, and possible spread from underlying cervical carcinoma occurring in some cases. Metastasis in such patients is still rarer. We present an elderly multiparous patient with previously diagnosed de-novo transitional-papillary primary endometrial squamous cell carcinoma (SCC) with large areas of ichthyosis uteri and severe dysplasia. Examination showed a firm to hard frontal forehead and scalp swelling. She underwent a radical hysterectomy six years ago for the same. She was advised radiotherapy, which she discontinued. Fine needle aspiration cytology (FNAC) of the forehead swelling revealed a metastatic SCC.
Keywords
Dysplasia
Ichthyosis uteri
Metastasis
Rare
SCC
INTRODUCTION
Focal transformation of one mature epithelium to another is a relatively common type of endometrial metaplasia. However, complete replacement with plaque-type squamous metaplasia is rarely seen and is labeled as Ichthyosis uteri. In literature, it has essentially been noted as an entity that does not turn malignant develops after long standing cervical obstruction or chronic inflammation.1 However, we found not only a primary malignancy developing in the backdrop of Ichthyosis uteri but also distant metastatic disease.
CASE REPORT
We are sharing a less documented case of upfront endometrial squamous cell carcinoma (SCC) in a pre-existing setting of extensive Ichthyosis uteri presenting with metastatic disease.
An elderly, multiparous lady presented to the oncology out patient department with a progressive, non-tender scalp swelling along with prolonged weakness and a persistent loss of appetite and weight. She attained menopause 12 to 15 years ago. Her history revealed that she underwent radical hysterectomy for post-menopausal bleeding, six years ago. This bleeding was diagnosed as SCC endometrium, showing areas of Ichthyosis uteri, in our department. The patient was thoroughly examined for metastatic disease by non-contrast enhanced computed tomography (NCCT) and fine needle aspiration cytology (FNAC) of the scalp swelling [Figure 1]. Metastatic work-up was done post the NCCT and FNAC. This included ultrasonography of the whole abdomen and a chest X-ray. Both were negative for metastasis. Due to financial constraints of the patient, a positron emission tomography-computed tomography could not be done.
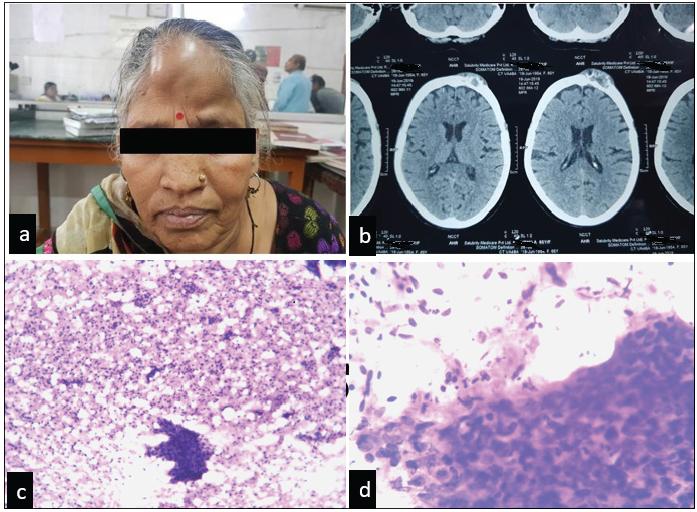
- (a) Patient presenting with frontal swelling. (b) NCCT showing well-defined, heterodense osteodestructive soft tissue lesion involving the frontal bone on the left side with destruction of the outer table of the skull and involvement of the overlying scalp. (c, d) FNAC findings of scalp swelling. H & E stained smears showings clusters of malignant squamous epithelial cells. (c) (4x), (d) (10x). NCCT:Contrast enhanced computed tomography, FNAC: Fine needle aspiration cytology, H&E: Hematoxylin and eosin stain.
FNAC was done, and smears were stained with hematoxylin and eosin. Smears were cellular, showing scattered and clustered atypical squamous cells. These cells exhibited increased nucleocytoplasmic ratios, hyperchromatic nuclei, moderate degrees of pleomorphism, and abundant amounts of eosinophilic cytoplasm [Figure 1]. Her previous records in the department were examined and reviewed.
Grossly, the specimen measured 11 x 5 x 4 cm. The cut section revealed a thickened and dilated endometrial cavity and endocervical canal. The cervix showed no obvious growth. The bilateral adnexa were grossly unremarkable.
Sections from the uterus showed the endometrium to be completely replaced by stratified squamous epithelium in a transitional-papillary architecture. Individual tumor cells showed distinct features depicting the malignant transformation. Polarities of the nuclei were lost, chromatin was intense, nuclear sizes were remarkably increased, and nuclear membranes were irregular. There were foci of invasion by these atypical cells into the superficial myometrium [Figure 2].
![(a, b) [H&E] Sections from endometrium showing micro-invasive squamous cell carcinoma. (4x,4x), (c) [H&E] Sections from cervix showing dysplasia (10x), H&E: Hematoxylin and eosin stain](/content/166/2025/0/1/img/ANAMS_58_2024-g2.png)
- (a, b) [H&E] Sections from endometrium showing micro-invasive squamous cell carcinoma. (4x,4x), (c) [H&E] Sections from cervix showing dysplasia (10x), H&E: Hematoxylin and eosin stain
Sections from the cervix showed moderate to severe dysplasia limited to the lower two-thirds of the cervical epithelium. The underlying stroma showed chronic lymphoplasmacytic infiltrate. The bilateral adnexa were free from tumor infiltration. The cervix was amputated and sampled for any invasion. However, there was no evidence of any invasive cervical malignancy [Figure 2]. Since the morphology was clear (by consensus), it was decided that immunohistochemistry had a limited role in differentiating primary and metastatic SCCs, so it was not attempted.
Hence, the diagnosis of a metastatic SCC from a primary SCC arising in Ichthyosis uteri was rendered.
Treatment
Post-hysterectomy, the patient was lost to follow-up. She only returned with the scalp swelling, which was diagnosed as a metastatic SCC for which she was given palliative chemotherapy comprising paclitaxel and carboplatin.
DISCUSSION
The endometrium exhibits a spectrum of epithelial metaplasia. Squamous metaplasia in the endometrium is seen in two forms: typical squamous and morular metaplasia. Typical squamous metaplasia shows features of keratin pearl formation with invasion and eosinophilia due to keratin production. Morular metaplasia or squamous morules are named so due to ball-like formations, composed of berry-like sheets of cells occupying the lumen of endometrial glands.1
We may encounter these more in cases of endometrial hyperplasia, sometimes in adenocarcinomas, and rarely in inflammation and obstruction leading to polyp formation. However, in extremely rare incidences, extensive parts of the endometrial lining may be completely transformed into ichthyosis uteri.2
The other names existing in literature for this entity are leukoplakia epidermidization, psoriasis uteri, epidermoid heteroplasia, cholesteometra, and indirect regenerative squamous metaplasia.3
Many etiological factors have been associated with this condition, though none have been clearly understood. Infestation, injury, and nutritional deficiencies are some of them.4 Ichthyosis uteri, although sporadically used in literature, has largely been considered banal. However, few sporadic cases show anaplastic and dysplastic changes in this setting.
The incumbent case was reported six years after a hysterectomy when the patient developed a metastatic disease in the scalp. Something that has never been reported in literature.5
Murhekar et al. (2008) and his team worked on a case quite like this one. The cervix showed similar moderate to severe dysplastic changes, while one of the tubes had a mucosal lining undergoing dysplasia.6 Fadare et al. (2006) described a dysplastic cervix with squamous changes in the endometrial glandular epithelia. The plausible explanation in this case was the proximal spread of human papillomavirus (HPV), which could have been present beforehand in the dysplastic area.7
Our case was similar to that of Murhekar et al. (2008),6 as the role of HPV could not be established from the, howsoever, coherent composite findings in the cervix and uterine corpus. Reasons for this deduction are: 1) Upon gross examination, no obvious growth in the cervix was discernible. 2) Serial sectioning of the cervix did not reveal any focus of invasion. 3) Menstruation had already ceased 4) The endometrial lining was hyperplastic in architecture, showing invasion.
Bagga et al. (2008) and Takeuchi et al. (2012) also reported similar presentations in females but with pus-filled pelvic cavities, variable dysplasia in the ectocervix, and endometrial carcinomas proven to be of squamous origin.3 Exploratory laparotomy was performed in each case, and histologic findings revealed an invasive SCC with lymph node metastasis associated with Ichthyosis uteri. The cervix showed no evidence of dysplasia, contrary to our case, where we noted moderate dysplasia.8
Bhardawaj N et. al. (2017) found an endometrioid type of adenocarcinoma with overlying extensive squamous metaplasia in one of their gynecologic patients.2 Bewtra C et al. (2005) similarly found Ichthyosis uteri in the fundus region.9
Conclusion
-
Ichthyosis uteri is a rare and interesting entity that should be considered while evaluating the uterus and cervix for malignancies.
-
Although the disease has been considered benign in literature, it can show malignant potential and can metastasize to distant sites.
Authors’ contributions
PM: Manuscript draft preparation and compilation of photographs; RJ: Diagnosis, differentials, correspondence and corrections.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Benign diseases of the endometrium: Blaustein’s pathology of female genital tract (7th ed). New York: Springer; 2019. p. :412-3.
- Ichthyosis uteri associated with endometrial adenocarcinoma: A case report. J Clin Diagn Res. 2017;11:ED24-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Primary endometrial squamous cell carcinoma with extensive squamous metaplasia and dysplasia. Indian J Pathol and Microbiology.. 2008;51:267-8.
- [CrossRef] [Google Scholar]
- Ichthyosis uteri complicated by poorly differentiated endometrial adenocarcinoma with squamous differentiation. Menopause Rev. 2013;6:449-52.
- [CrossRef] [Google Scholar]
- Does “ichthyosis uteri” have malignant potential? A case report of squamous cell carcinoma of endometrium associated with extensive ichthyosis uteri. Diagn Pathol. 2008;3:4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dysplastic ichthyosis uteri-like changes of the entire endometrium associated with a squamous cell carcinoma of the uterine cervix. Diagn Pathol. 2006;1:8.
- [CrossRef] [PubMed] [Google Scholar]
- A case of primary squamous cell carcinoma of the endometrium associated with extensive “ichthyosis uteri”. Eur J Gynaecol Oncol. 2012;33:552-4.
- [PubMed] [Google Scholar]
- Ichthyosis uteri: A case report and review of the literature. Arch Pathol Lab Med. 2005;129:e124-5.
- [CrossRef] [PubMed] [Google Scholar]





