Translate this page into:
Molecular Genetic Analysis of Mycobacteria, Causing Female Genital Tuberculosis: Possibilities of Sexual Transmission–An Overview
Address for correspondence Asesh Banerjee, PhD, Amity Institute of Biotechnology, Amity University Kolkata, Action Area II, Rajarhat Newtown, Kolkata, West Bengal 700135, India (e-mail: abanerjee1@kol.amity.edu).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Although tuberculosis (TB) is predominantly known to be a traditional air-borne disease, new modes of transmission have also come to light. While the lungs remain the main entry point, TB can spread to other regions of the body causing extrapulmonary tuberculosis (EPTB). Female genital tuberculosis is one such EPTB that can adversely affect females between the ages of 15 and 45 years and may cause hindrance in their ability of conception and successful pregnancy. Sexual transmission of TB is a lesser-known or poorly investigated route of spread that has recently been confirmed through molecular evidence. Targeted molecular-level studies by polymerase chain reaction (PCR), in addition to interim diagnostic techniques, have offered evidence for the sexual transmission of Mycobacterium subtypes. Recent studies conducted using multiplex PCR on both the male and female counterparts revealed that the male partners had Mycobacterium in their semen, while the female counterparts had it in their endometrium and products of conception resulting in miscarriage. These studies indicate that the mycobacterial infection/infestation in the females may have been brought on by contact with infected male semen. Therefore, it is necessary to identify the genetic loci that are responsible for the sexual transmission of mycobacteria. This can be done by whole-genome sequencing. It has also to be emphasized that screening of sexually active males for genital TB in endemic regions is necessary for the prevention of sexual transmission of mycobacteria.
Keywords
mycobacteria
genital tuberculosis
infertility
multiplex-PCR
sexual transmission
genome sequencing
Introduction
Tuberculosis (TB) is one of the aerosol-borne infections caused mainly due to Mycobacterium tuberculosis (MTB). During the past few centuries, dissemination of MTB was established as extrapulmonary tuberculosis (EPTB). Genital TB (GTB) is one of forms of EPTB.1 It primarily reaches the genital areas via dissemination from foci outside the genitalia with the lungs being the original seeding site.2 Males are affected with GTB mainly at a reproductive age between 29 and 32 years.3 The semen (seminal fluid), in contact with the infected scrotal parts, gets infected and carries the nonmotile MTB along with it. Epididymis (45%) and prostate (22–49%) get mostly infected, whereas the chances of testes infection are comparatively much less (3%).4 In the case of females, a high risk of infertility may result in recurrent miscarriage, ectopic pregnancies, and menstrual irregularities. MTB often damages the fallopian tube (90–100%), endometrium (50–60%), ovary (20–30%), cervix (5–15%), and vagina (1%). The disease is most prevalent between 15 and 45 years.5 Infertility in females may be due to sexual transmission of MTB from an affected male through infected semen. The theoretical possibilities of sexual transmission of MTB from one mucosal surface to another have been described in animal models during intercourse.6 But the sexual transfer of TB and nontuberculous mycobacteria (NTM) in humans has rarely been found and only anecdotally reported.7 Here, we are going to review the possibilities of sexual transmission of both MTB and NTM from male to female.
Epidemiology
GTB is serious clinical condition that can often be asymptomatic. It can mostly go unnoticed or masquerade as other gynecological conditions.8 Asia Pacific region has 58% of the global burden of TB.9 EPTB accounts for 27% of TB cases worldwide, of which 9% is GTB. The global distribution of GTB varies according to geographical location: 15 to 20% in Asia, Africa, Eastern Europe and the Russian Federation; 2 to 10% in the United States and Western Europe.10 Recent studies have shown an incidence of 3 to 16% female genital tuberculosis (FGTB) in Indian patients registered for infertility. Further studies with women inscribed for in vitro fertilization from North India reported the prevalence of FGTB in 48.5% of tubal factor infertility cases.11 Indian Council of Medical Research has reported that the prevalence of FGTB in India has amplified from 19% of EPTB in 2011 to 30% in 2015.12
Diagnosis
Highly sensitive diagnostic modalities, such as polymerase chain reaction (PCR), molecular typing, enzyme-linked immunosorbent assay (ELISA), and whole-genome sequencing (WGS), are performed to detect mycobacterial DNA in male partners of female patients with suspected TB. PCR is a rapid, sensitive, and specific molecular method that targets various gene segments in the MTB DNA.13 However, this technique has chances of giving false-positive reports.14 Multiplex PCR can be performed on various TB samples using more than two primer pairs. This results in a highly specific and sensitive diagnosis of FGTB than the other conventional PCR technique.15,16
Molecular typing is one of the powerful tools used for identifying specific mycobacteria strains.17 The identification and presence of the organism are confirmed by restriction fragment length polymorphism that is based on the insertion element.18,19
ELISA has shown to be significantly effective in detecting GTB compared to laparoscopy. Even in cases where the culture is found to be negative, ELISA can successfully detect the sensitivity of antimycobacterial antibodies immunoglobulin M and immunoglobulin G.20 However, at present, it is not considered specific and sensitive. Hence, ELISA was banned by the World Health Organization and Government of India for diagnosis of GTB.21 A summary of methods associated with detection of FGTB is illustrated in ►Fig. 1.
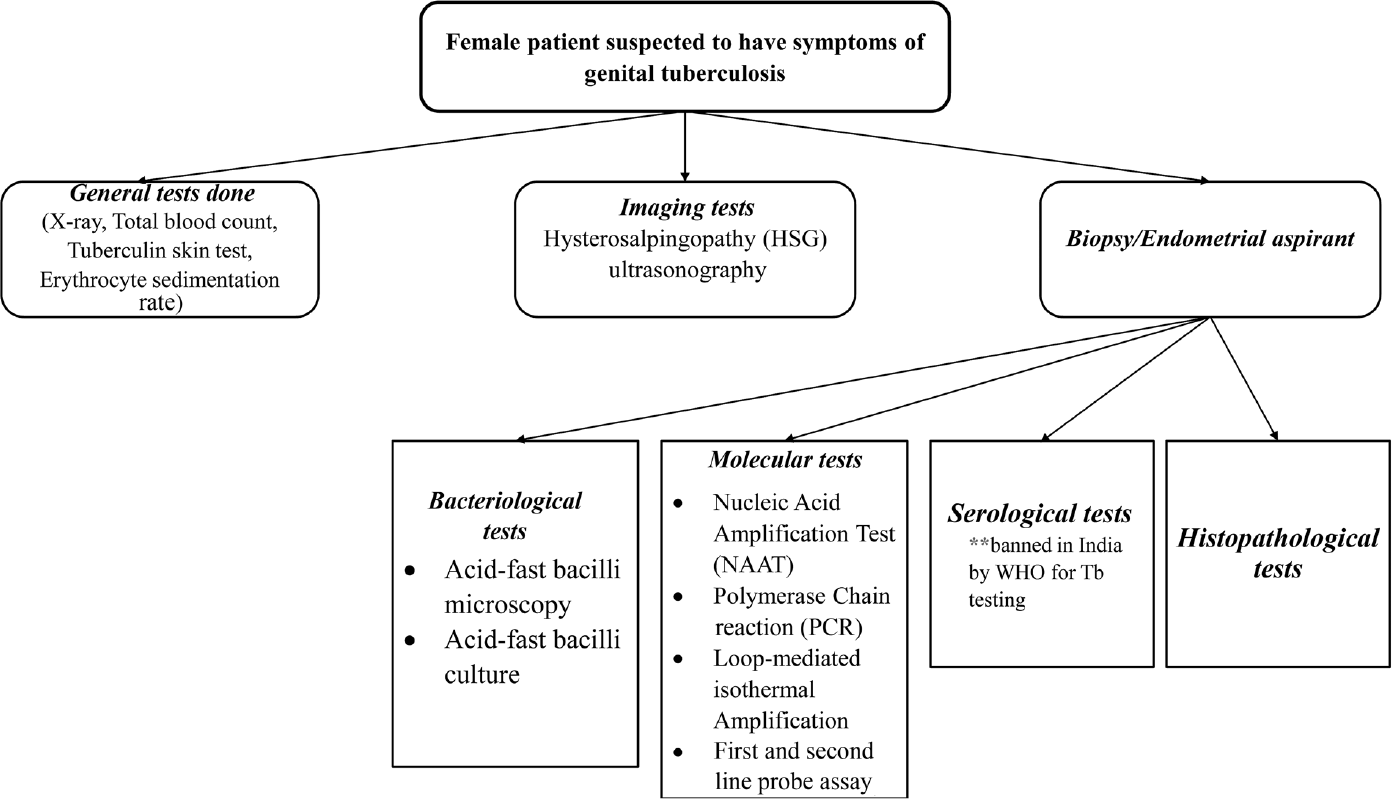
- Detection of female genital tuberculosis.
GTB is known to be one of the causes of female infertility with an incidence rate of 44-74% worldwide with more than 50% of cases reported in India. Due to the asymptomatic nature of latent genital tuberculosis, it often gets undiagnosed and/or misdiagnosed causing hindrance in the prediction of the accurate prevalence of the condition.22 Because of its asymptomatic nature, it may mimic or coexist with other gynecological and abdominal disorders, making its diagnosis difficult.12 In cases of fertility issues, the presence of tubercular bacilli in the genital tract is a critical factor because the endometrium becomes nonreceptive, restricting any implantation or rejection of implanted embryos in the initial months, resulting in recurrent pregnancy loss.23 Several studies have shown that women who have TB are at greater risk of facing adverse pregnancy outcomes such as stillbirth, low-birth weight, and premature birth, than women who are not suffering from TB.24,25 In clinical laboratories, traditionally, MTB is diagnosed either by acid-fast staining or can be grown on a Lowenstein–Jensen (LJ medium). But both cases have a set of disadvantages. In the first case, the microscopic observation of acid-fast bacilli is sensitive and requires a minimum of 10,000 organisms ml−1 in the sample. However, the growth on the LJ medium takes up to a week to develop.26 Moreover, a considerable number of lesions found in the genital tract are seen to be bacteriologically mute. Owing to the drawbacks of the mycobacteriological and histopathological tests, their use in the diagnosis of GTB is restricted.13 For analyzing and identifying both pulmonary TB (PTB) and EPTB, PCR-based studies have shown improved sensitivity, speed, and specificity. According to a blind study conducted by Bhanu et al on 61 female patients, use of amplification of the mpt64 gene segment demonstrated increased sensitivity in the diagnosis of FGTB.13 Another infertility study related to FGTB has shown that even though hysterosalpingography and laparoscopy are used for the diagnosis of FGTB, a more molecular level technique such as PCR yields higher rates of specificity and sensitivity.22
Known Transmission Routes and Evidence of Sexual Transfer
From the early times of human civilization, TB was considered an exclusively air-borne pathogenic pulmonary infection. The lung is the main entry point of the disease; TB can also spread to other organs like the brain and bone, causing EPTB.27 Lübeck disaster that occurred around 1929 to 1930 in Germany was one such example that pointed toward the transmission of Mycobacterium through ingestion. Among the 251 neonates who were given the oral Bacillus Calmette–Guérin vaccine contaminated with various inoculum of the virulent MTB Kiel strain, 90% developed clinical TB, whereas 13% developed PTB and 28.7% succumbed to death.28 The lesser-known mode of transmission via the sexual route was not a popular hypothesis until meaningful molecular evidence was found in early 2000.7,18 This was because usual pathogens that cause sexually transmitted diseases (STDs) are known to have the characteristic of being “fast and loose.” They can usually change the surface antigen thus using this as a strategy to dodge the host immune response. They are also fast growing and hence can generate altered or mutated populations rapidly. This helps in colonization of the lower genital niches and subsequent upward migration.
Mycobacteria are the archetype of “slow and steady” and cannot change the surface quickly. Their genomes are relatively inert and, on the whole, they are slow growing. These properties do not fit well with the general characteristics of the STD-causing bacteria that colonize the lower niches of the genitalia.
Angus et al in 2001 observed that the MTB subtypes found in the female with endometrial TB were identical to the isolates found from the skin ulcers on the penis of the infected male counterpart.18 In the study conducted by Kimura et al in 2018, it was discovered that organisms in both the male and female isolates were genotypically identical in one of the couples.29 The study further demonstrated through WGS that the isolates from both the individuals had only one base pair difference. What was observed from both studies was that, the male counterpart was initially affected with TB. Then, after a period of time, the female was also infected. This strengthened the idea that the transmission was from male to female.
In 2021, a cohort study was done in mainland China with a total of 3,668,004 women along with their male partners. This study observed that women with partners who have TB were 2.13 times more likely to give stillbirth than women whose partners were not affected, making TB one of the risk factors causing stillbirth in pregnancy.30
A multiplex PCR-based study carried out by Datta et al in 2022 at the Calcutta Fertility Mission reported 165 couples with primary and secondary infertility between 2019 and 2020.31 In this study design, the couples with known causes of infertility such as tubal factors (22 pairs), endometriosis (17), Polycystic Ovarian Disease (14), Pelvic Inflammatory Disease (11), Hormonal imbalance (10), history of pulmonary or EPTB (6), history of Azoospermia (2), and local bacterial infection in genitalia (2) were screened and excluded. The remaining 81 sexually active couples with unknown causes of infertility, normal functioning fallopian tube and hormonal profile, and absence of any local bacterial infection were included. All the couples were screened for mycobacterial DNA using the multiplex PCR. The couples were then segregated into group A (40), B (20), and C (21). In groups A and B, both the partners tested positive for GTB but were asymptomatic. However, group C was assigned as the negative control since all the couples in this group were tested negative for GTB. The main difference between the groups A and B was that, in group B, the female partner's endometrium was tested positive for GTB. On the contrary, in group A, the product of conception tested positive for GTB after miscarriage, but their endometria were tested negative. It was further observed that the couples were tested positive for three combinations of mycobacteria: two strains of MTB and one NTM strain. Whichever strain(s) were found in the male partner, they have shown a transfer rate of 100% to his female counterpart. Thus, for the first-time, a cohort-based reporting was done on large-scale sexual transmission of mycobacteria. A schematic diagram of sexual transfer of mycobacteria is illustrated in ►Fig. 2. This study also reported that the sperm quality or motility was not a factor in the failure of pregnancy.31

- Schematic illustration for sexual transmission of Mycobacterial spp..
Conclusion
Several studies have offered substantial evidence for the likelihood of sexual transmission of TB over the years, but it is still an area that needs further exploration. Understanding the pathogenesis of FGTB is critical for establishing a link between female infertility and the transfer of Mycobacterium from male semen to female via sexual transmission. 16s rDNA sequencing, followed by Genome Wide Association Studies of mycobacteria involved in FGTB, is needed to successfully identify the genetic determinants that are responsible for its sexual transfer. It also will reveal genetic factors behind lower genital colonization in women. Further, this will lead to the discovery of MTB/NTM types that are sexually transferred. Since GTB in male can often be asymptomatic, multiplex PCR using the semen samples can provide a tool for identifying the risk of infertility. Additionally, a public health clause for screening of sex partners in countries with a high incidence of TB can also be suggested.
Authors' Contribution
S.C., S.K., and S.G. were involved in manuscript writing, main draft and revised draft. P.G. contributed to manuscript planning, remodeling, and revision. P.S., S.N., A.D., and S.C. helped in manuscript writing, main draft. A.B. was involved in manuscript planning, writing, and revision; main draft and revised draft.
Acknowledgement
We are thankful to Diya Adhikary, Srijan Dubey, and Ankuri Chakraborty, for helping in the preparation of the manuscript
Conflict of Interest
None declared.
References
- Genital tuberculosis and its impact on male and female infertility. US Endocrinol. 2020;16(02):97.
- [CrossRef] [Google Scholar]
- Isolated tuberculous epididymitis: a review of forty cases. J Postgrad Med. 2005;51(02):109-111. discussion 111
- [Google Scholar]
- A study on genital tuberculosis and infertility in Indian population. Arch Med (Oviedo). 2010;2(01):1.
- [Google Scholar]
- Ansteckungsmöglichkeit der TuberkulosedurchKohabitationimTierexperiment. [Experimental study on the possibility of tuberculosis transmission by coitus] Z Urol Nephrol. 1979;72(12):911-914.
- [Google Scholar]
- Possible sexual transmission of genitourinary tuberculosis. Int J Tuberc Lung Dis. 1998;2(05):439.
- [Google Scholar]
- Genital tuberculosis: current status of diagnosis and management. Transl Androl Urol. 2017;6(02):222-233.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculosis and gender in the Asia-Pacific region. Aust N Z J Public Health. 2017;41(03):227-229.
- [CrossRef] [PubMed] [Google Scholar]
- Urogenital tuberculosis - epidemiology, pathogenesis and clinical features. Nat Rev Urol. 2019;16(10):573-598.
- [CrossRef] [PubMed] [Google Scholar]
- Genital tuberculosis: a leading cause for infertility in women seeking assisted conception in North India. Arch Gynecol Obstet. 2008;278(04):325-327.
- [CrossRef] [PubMed] [Google Scholar]
- Improved diagnostic value of PCR in the diagnosis of female genital tuberculosis leading to infertility. J Med Microbiol. 2005;54(Pt 10):927-931.
- [CrossRef] [PubMed] [Google Scholar]
- Relevance of semen polymerase chain reaction positive for tuberculosis in asymptomatic men undergoing infertility evaluation. J Hum Reprod Sci. 2015;8(03):165-169.
- [CrossRef] [PubMed] [Google Scholar]
- Development of a new sensitive and efficient multiplex polymerase chain reaction (PCR) for identification and differentiation of different mycobacterial species. Trop Med Int Health. 2003;8(02):150-157.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of multiplex PCR for rapid diagnosis of female genital tuberculosis. J Assoc Physicians India. 2019;67(12):21-24.
- [Google Scholar]
- Current methods in the molecular typing of Mycobacterium tuberculosis and other mycobacteria. BioMed Res Int. 2014;2014:645802. DOI: 10.1155/2014/645802
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous tuberculosis of the penis and sexual transmission of tuberculosis confirmed by molecular typing. Clin Infect Dis. 2001;33(11):E132-E134.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of strategies for molecular fingerprinting for use in the routine work of a Mycobacterium reference unit. J Clin Microbiol. 1998;36(11):3385-3388.
- [CrossRef] [PubMed] [Google Scholar]
- Role of ELISA (enzyme-linked immunosorbent assay) in genital tuberculosis. Int J Gynaecol Obstet. 1997;57(02):205-206.
- [CrossRef] [PubMed] [Google Scholar]
- Role of latent genital tuberculosis in repeated IVF failure in the Indian clinical setting. Gynecol Obstet Invest. 2006;61(04):223-227.
- [CrossRef] [PubMed] [Google Scholar]
- Role of latent female genital tuberculosis in recurrent early pregnancy loss: a retrospective analysis. Int J Reprod Biomed (Yazd). 2019;17(12):929-934.
- [CrossRef] [PubMed] [Google Scholar]
- Increased risk of low birthweight and small for gestational age infants among women with tuberculosis. BJOG. 2010;117(05):585-590.
- [CrossRef] [PubMed] [Google Scholar]
- Medical and obstetric outcomes among pregnant women with tuberculosis: a population-based study of 7.8 million births. Am J Obstet Gynecol. 2016;215(06):797.e1-797.e6.
- [CrossRef] [PubMed] [Google Scholar]
- What we know about tuberculosis transmission: an overview. J Infect Dis. 2017;216(suppl_6):S629-S635.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculosis in Newborns: The Lessons of the “Lübeck Disaster” (1929. PLoSPathog. 2016;12(01):e1005271. Published 1933;2016(Jan):21 DOI: 10.1371/journal.ppat.1005271
- [CrossRef] [PubMed] [Google Scholar]
- First case of sexually transmitted asymptomatic female genital tuberculosis from spousal epididymal tuberculosis diagnosed by active screening. Int J Infect Dis. 2018;73:60-62.
- [CrossRef] [PubMed] [Google Scholar]
- Increased risk of stillbirth among women whose partner has tuberculosis. BioMed Res Int. 2021;2021:1837881. Published 2021 Sep 14. DOI: 10.1155/2021/1837881
- [CrossRef] [PubMed] [Google Scholar]
- Evidence of sexual transfer of mycobacteria from male to female partners reporting to an IVF clinic. Trop Doct. 2022;52(02):331-334.
- [CrossRef] [PubMed] [Google Scholar]






