Translate this page into:
NAMS task force report on antimicrobial resistance
Corresponding author: Prof. Arunaloke Chakrabarti, MD, Department of Infectious Disease, Doodhadhari Burfani Hospital and Research Institute, Bhupatwala, Haridwar, India. arunaloke@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chakrabarti A, Balaji V, Bansal N, Gopalakrishnan R, Gupta P, Jain A, et al. NAMS task force report on antimicrobial resistance. Ann Natl Acad Med Sci (India). 2025;61:171-209. doi: 10.25259/ANAMS_TFR_13_2024
EXECUTIVE SUMMARY
Background
Antimicrobial resistance (AMR) has emerged as a public health concern, especially in light of the fact that newer antibiotic classes have been slow to develop and investments in novel antimicrobial drug classes have been receding. India is among the countries that contribute significantly to global AMR due to extensive antibiotic abuse, a prime driver of AMR. Widespread resistance increases the use of broad-spectrum empiric antibiotic therapy, narrowing treatment options and worsening patient outcomes. Over-the-counter use of antibiotics, lack of awareness, inadequate use of diagnostics, overcrowding, cross-infections, financial compensation of doctors by pharmaceuticals, and poor health infrastructure also amplify AMR problems in India. Referred to as the world’s AMR capital, India is battling emergent superbugs with limited treatment options. In India, annually, more than 58,000 newborns die due to sepsis triggered by resistant bacteria, which is expected to rise to 2 million deaths by 2050.
Current antimicrobial resistance scenario in India
The available data indicates a rise in the AMR rates across multiple pathogens of clinical importance. An indicator of the rising tide of AMR in India is the rapidly increasing proportion of isolates that are resistant to extended-spectrum cephalosporins and carbapenems. Among Enterobacterales, >70% of Escherichia coli and at least 80% of Klebsiella pneumoniae were extended-spectrum beta-lactamase producers. A substantial level of carbapenem resistance has been reported in K. pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii. In the past 6 years, carbapenem resistance has substantially increased in hospital-acquired isolates of both E. coli (from 19% in 2017 to 34% in 2022) and K. pneumoniae (from 41% in 2017 to 58% in 2022). More than 30% of P. aeruginosa isolates and >90% of A. baumannii isolates are resistant to carbapenems. Carbapenem-resistant A. baumannii is the leading cause of ventilator-associated pneumonia in Indian intensive care units (ICUs).
In India, a sharp increase in carbapenem resistance in gram-negative pathogens leaves patients with limited treatment options and increases the risk of AMR-attributed mortality in patients. Emergence of resistance to colistin has already been documented in India, although current resistance rates are estimated to be less than 10%. In addition, emerging new resistance mechanisms such as PBP3 insert in E. coli and mutation in the siderophore iron transport channels contributing to the development of pan-drug resistance are of great concern. As India has been witnessing a dominance of New Delhi metallo-β-lactamase (NDM) among Enterobacterales, clinicians are desperately looking for safer and effective substitutes for polymyxins that are currently considered as salvage therapies. Among gram-positive pathogens, there is an incremental increase in the trend of Methicillin resistant staphylococcus aureus (MRSA), 33% in 2017 to 44.5% in 2023. Similarly, there is a noticeable increase in the proportion of vancomycin resistance in Enterococcus sp., which is five times higher in E. faecium than E. faecalis.
The Indian Council of Medical Research (ICMR) has established a national network on surveillance of AMR in laboratories based at academic centers, targeting medically important index microbes that have been identified by the World Health Organization (WHO). The Antimicrobial Resistance Surveillance Research Network (AMRSN) established by the ICMR has six reference laboratories for six pathogenic groups that are located in four tertiary care medical institutions. The AMRSN also incorporates an in-depth understanding of the clonality of drug-resistant pathogens and the transmission dynamics to enable a better understanding of AMR in the Indian context and devise suitable interventions. However, more proactive steps are warranted. The burden of AMR in community, livestock, and food animals has been poorly documented in India. Apart from sporadic, small, localized studies, evidence that can be extrapolated to the national level is lacking. The AMRSN, although currently limited to human health, plans to scale up on a national level and expand its ambit to include samples from a wider spectrum of sources, including animal, environmental, and food samples, to reflect the One Health approach to surveillance. Apart from the absence of a One Health approach to surveillance, another weakness of the existing surveillance systems for AMR in India is that it does not account for antibiotic use.
The existence of a surveillance system that can establish the relationship between antibiotic consumption patterns and the emergence of AMR is vital in producing evidence that may help in the designing and evaluation of effective interventions. In 2017, the Indian Ministry of Health and Family Welfare published the national action plan (NAP) for containing AMR. This 5-year NAP on AMR (2017–2021) outlines the priorities and implementation strategies for curbing AMR in India. NAP focuses equally on human, environment, and food–animal sectors to encompass a One Health approach. Priorities outlined in the NAP for antimicrobial resistance in India are as follows: i) improve awareness and understanding of AMR through effective communication, education, and training; ii) strengthen knowledge and evidence through surveillance; iii) reduce the incidence of infection through effective infection, prevention, and control; iv) optimize the use of antimicrobial agents in all sectors; v) promote investments for AMR activities, research, and innovations; and vi) strengthen India’s leadership on AMR by means of collaborations on AMR at international, national, and subnational levels. However, implementation is slow and a big push is needed by all stakeholders. The lack of a separate financial allocation remains the greatest challenge for the implementation of NAPs in India.
Antifungal resistance was never a major concern till the emergence of multidrug-resistant Candida auris. This resistant fungus entered Indian ICUs in 2009 and has since been isolated in nearly all hospitals in India. This fungus behaves like a bacterium, develops resistance very quickly, is easily transmitted, causes outbreaks, contaminates the hospital environment, resists many disinfectants, and is not easily identified. Indian strains are classified under Clade I, which has resistance rates of 58–100% to fluconazole, 50% to voriconazole, 30% to amphotericin B, and 10% to at least one echinocandin. At present, C. auris is the most common yeast fungus isolated from blood in many Indian ICUs. Besides C. auris, fluconazole resistance is increasing in the commonly isolated species such as C. tropicalis and C. parapsilosis in many centers of this country. Among mycelial fungi, though azole resistance is not very high in Aspergillus fumigatus in India, the comparatively more resistant A. flavus is isolated as often as A. fumigatus from patients with invasive aspergillosis.
Management of dermatophytosis was never considered difficult until the emergence of Trichophyton indotineae, a new species of the T. mentagrophytes complex, which is resistant to multiple antifungal agents, especially allylamines, across India.
Gaps in addressing antimicrobial resistance
Unnecessary or irrational use of antibiotics in humans, animals, and agro-industries and inadequate diagnostic facilities are among the leading causes of AMR. There is a lack of national-level data on the overuse or misuse of antibiotics in the community, animals, environment, and agriculture. The inappropriate use of colistin in animals as a growth promoter and in agriculture has led to colistin resistance in India. Given that there are few regulations against the use of antibiotics for non-therapeutic purposes in India, with no stringent implementation protocols even when there are regulations, the emergence of AMR from antibiotic overuse in the animal sector is likely to be an unmeasured burden in India.
Lack of trained manpower and inadequate laboratory facilities to support clinicians with the microbial culture test and suggesting an appropriate antibiotic may result in inappropriate antibiotic use. Moreover, India lacks diagnostic facilities to identify resistant pathogens’ susceptibility to antibiotics. Respiratory tract infections are common, and improved diagnostics could substantially reduce antibiotic overuse.
Rapid diagnostic tests are quick, easy to perform, highly sensitive, and specific in point-of-care settings, thereby facilitating timely definitive therapy and reducing length of stay, cost, morbidity, and mortality. This is further compounded by the inadequacy of healthcare facilities lack of monitoring systems to control antibiotic prescription and dispensing practice by health system (to stop the sales of over-the-counter antibiotics).
India is also known for poor antibiotic stewardship practices that are reflected in the high antibiotic resistance rate. Inadequate training on antimicrobial prescription combined with the lack of a functional policy on antimicrobial use has led to unchecked growth of AMR in Indian hospitals. AMSP program at the Indian healthcare level is still rudimentary and needs to gain momentum to develop proper disease surveillance infrastructure and initiate basic AMR research. Structured education and training on AMSP are inadequate in India. A survey found that 88% of postgraduate students did not receive any education and training at induction or during employment. The intervention of various stakeholders is essential for the sustainable design and implementation of hospital-based AMSP in India.
Inadequate infection prevention control practices, including hand hygiene promotes the transmission of AMR.
Increasing awareness and understanding of AMR through effective communication, education, and training is one of the main strategies to contain AMR. It has to leverage public communication programs to encourage behavior change in target populations, namely, stakeholders in human and animal health, and agriculture. There is also a need to increase awareness about the necessity to contain AMR at higher levels of policymaking, so that this aspect may emerge as a priority in the health policies of the nation. In addition, the exit of big pharma from antibiotic development and the lack of investment from venture capitalists to support the commercial viability of antibacterial agents have pushed AMR into a global health crisis. Without addressing gaps in the identified areas, sustained progress in AMR mitigation is unlikely.
Recommended intervention strategies
Tackling AMR requires a comprehensive set of interventions. Health workers across a spectrum of disciplines play an important role in ensuring the responsible use of antimicrobial agents to treat prevalent infectious diseases. Simple measures to prevent infections, such as vaccinations and promoting hand hygiene and better hygiene in healthcare facilities, more than halve the risk of death and decrease the health burden of AMR. Similarly, integrated delivery of policies that promote hospital hygiene, antimicrobial stewardship (AMS), and the use of diagnostic tests to differentiate bacterial versus viral infections and mass media campaigns could significantly reduce the burden of drug-resistant infections. WHO defines AMS as a coherent set of integrated actions that promote the responsible and appropriate use of antimicrobials to help improve patient outcomes across the continuum of care. Moreover, effective implementation of AMS activities in healthcare facilities require a comprehensive approach at national policy and program levels.
Responsible and appropriate use of antimicrobials include prescribing only when needed and selection of the optimal drug regimen, drug dosing, route of administration, and duration of treatment following proper and optimized diagnosis. Evidence shows that health workers and students want to improve their knowledge and level of competency through targeted, effective, and relevant education and training on AMR. The module of the curricula guide includes the following: i) build knowledge and awareness of AMR; ii) appropriate use of antimicrobial agents; iii) infection prevention and control; iv) diagnostic stewardship and surveillance; and v) ethics, leadership, communication, and governance. In addition, public engagement and education aim to empower and engage the public on the risk of exposure to antimicrobials. Moreover, strengthening AMR surveillance improve the understanding of AMR, and how resistant microorganisms spread across and between humans, animals, agriculture, and the environment.
Many organizations have been formed and policies framed to control infectious diseases, optimize antibiotic usage, and prevent AMR. Between 2009 and 2011, the Global Antibiotic Resistance Partnership (GARP), India working group was established to create a platform for developing practical policy proposals on AMR. In 2012, ICMR initiated an antimicrobial stewardship program (AMSP) in collaboration with many institutions. In 2014, the Central Drug Standard Control Organization (CDSCO) introduced Schedule H1, in which antibiotics could be dispensed only against a valid prescription, with records of antibiotic sales to be maintained for at least 3 years. In 2016, the National Center for Disease Control (NCDC) published the National Treatment Guidelines for antimicrobial use in infectious diseases. Further, in 2017, ICMR published guidelines on the optimization of antibiotics, which includes the new WHO method of antibiotic classification (“AWaRe”) in the 20th Essential Medicine list to strengthen AMSPs in hospitals. The ICMR also instituted an evaluation of the AMSP through an in-depth facility survey in private and government healthcare institutions. It is observed that the stewardship programs in private institutions were better equipped to deal with emerging crises such as AMR or hospital-associated infection outbreaks, as compared to the government facilities in the survey. It is suggested that the accreditation mandates, which certain private institutes adhere to on account of financial compulsions, may have a positive impact on the program. The purpose of the document is to provide core interventions to mitigate AMR. The proposed interventions are based on the strategies at the level of policymakers, regulatory, and healthcare facilities, including antimicrobial stewardship and prevention of infections, clinical microbiology laboratory for timely and accurate diagnosis, research and development of novel antibiotics, and rapid diagnostics and one health approach. Undoubtedly, mitigating AMR requires a coordinated approach across the human and animal health, agriculture, and the environment sectors. In the long term, effective multisectoral collaboration requires governments to take ownership of the NAP implementation process and ensure it is appropriately resourced and given sufficient visibility to keep it a national priority. The document could be helpful to other stakeholders, such as those responsible for healthcare quality improvement, patient safety, health facility accreditation or regulation, public health, infectious disease control and surveillance, water, sanitation and hygiene, occupational health, AMSP, clinical microbiology, and environmental health interventions.
CURRENT STATUS OF ANTIMICROBIAL RESISTANCE IN INDIA
(a) Antibacterial resistance
Antimicrobial resistance (AMR) has been identified as a serious threat to global health, with an estimated 4.95 million deaths associated with bacterial AMR.1 The speed with which new resistance phenotypes and mechanisms has emerged and spread highlights that the development of new drugs alone is not sufficient to address the growing resistance problem. Two institutions within India’s Ministry of Health, the Indian Council of Medical Research (ICMR) and the National Center for Disease Control (NCDC), have developed national networks of public and private hospitals to measure AMR trends, prevent healthcare-associated infections (HAIs), and enhance appropriate use of antibiotics.
Gram-negative pathogens
In the study of national AMR surveillance conducted by ICMR and NCDC, Enterobacterales and non-fermenting gram-negative bacilli (P. aeruginosa and A. baumannii) were reported as the most common gram-negative pathogens.2,3 The AMR surveillance data for 2022, reported by ICMR and National Antimicrobial Surveillance network (NARS-Net) Net by NCDC, is given in Table 1. In E. coli, >70% of the isolates are phenotypically identified as Extended-spectrum beta-lactamase (ESBL)-producers; among them, CTXM-15 (34%) was the most common ESBL-encoding gene, followed by CTXM-1 (19%) and Temoniera extended spectrum beta-lactamase (TEM) (17%). An inhibitor-resistant penicillinase, OXA-1, was found in 28% of ESBL E. coli isolates. Among K. pneumoniae, at least 80% of the isolates were identified as ESBL producers, SHV (49%) was predominant, followed by CTXM-15 (34%) and CTXM-1 (23%). OXA-1 was identified in 22% of K. pneumoniae isolates. In the past 6 years, carbapenem resistance has substantially increased in both E. coli (from 19% in 2017 to 34% in 2022), and K. pneumoniae (from 41% in 2017 to 58% in 2022). Among carbapenem-resistant E. coli, NDM-1 was seen in >95% of the isolates.4 In carbapenem-resistant K. pneumoniae, dual carriage of both New Delhi metallo-β-lactamase (NDM) and OXA-48-like carbapenemases was found in 60% of the isolates, while OXA-48-like carbapenemases alone was seen in 40% of the isolates.5
| % of cephalosporin-resistant gram negative pathogens | ||||
|---|---|---|---|---|
| E. coli | K. pneumoniae | P. aeruginosa | A. baumannii | |
| ICMR | 81 | 81 | 41 | 91 |
| NARS-Net | 76 | 83 | 44 | 73 |
| % of carbapenem resistance in gram negative pathogens | ||||
| E. coli | K. pneumoniae | P. aeruginosa | A. baumannii | |
| ICMR | 30 | 56 | 36 | 86 |
| NARS-Net | 35 | 47 | 27 | 59 |
| % of colistin resistance in gram negative pathogens | ||||
| E. coli | K. pneumoniae | P. aeruginosa | A. baumannii | |
| ICMR | 3 | 6 | 3 | 5 |
| NARS-Net | 0 | < 1 (0.4) | < 1 (0.1) | < 1 (0.4) |
AMR: Antimicrobial resistance, ICMR: Indian Council of Medical Research, NARS: National Antimicrobial Resistance Surveillance Network (NARS-Net India)
Susceptibility to amikacin was significantly different between carbapenem-resistant E. coli (52%) and K. pneumoniae (16%) isolates. Recently, in 2022, the Clinical and Laboratory Standards Institute (CLSI) guideline has lowered the susceptibility breakpoint of amikacin to ≤ 4 mg/L: applying this revised breakpoint further reduces the susceptibility to amikacin.6 Further, the combination of aminoglycoside-modifying enzymes with 16SRMTases is observed in 48% of NDM-producing E. coli and 35% of OXA-48-like-producing K. pneumoniae.7 Therefore, the increasing prevalence of 16SRMTases limits the clinical utility of aminoglycosides including the new agent plazomicin against carbapenem-resistant Enterobacterales. Fluroquinolones (>60% of isolates are resistant) showed limited activity against Enterobacterales.2,3
The acronym SPICE (Serratia spp, Providencia spp, indole-positive proteus, Citrobacter spp., and Enterobacter spp.) denotes organisms at risk for ampC production. Indole-positive Proteus spp. currently refers to organisms such as P. vulgaris and P. penneri, which generally do not contain chromosomal ampC genes. The emergence of clinically relevant ampC expression during antibiotic treatment has been most frequently described in E. cloacae, K. aerogenes (formerly Enterobacter aerogenes), and C. freundii.8 Clinical reports suggest that the emergence of resistance after exposure to an agent like ceftriaxone may occur in approximately 8–40% of infections caused by these pathogens.9 Therefore, when E. cloacae, K. aerogenes, or C. freundii are recovered in clinical cultures (other than those associated with uncomplicated cystitis), treatment with ceftriaxone or ceftazidime is not recommended, even if an isolate initially tests susceptible to these agents.9 In contrast, other organisms historically presumed to be at risk for the development of clinically significant ampC expression, such as Serratia marcescens, Morganella morganii, and Providencia species, express clinically significant ampC production in less than 5% of isolates, and antibiotics can be selected according to susceptibility testing results.8
Enterobacter spp. and Citrobacter spp. are the most common pathogens, and the remaining are seen in ≤ 1% of the clinical cultures.2,3 Carbapenem resistance rates are lesser than 30% in the SPICE pathogens (27% in E. cloacae, 29% in C. freundii, 22% in C. koseri, and 23% in S. marcescens). In the past 6 years, susceptibility to piperacillin/tazobactam has been steadily decreased in Enterobacter spp. (62% in 2017 to 38% in 2022) and Citrobacter spp. (58% in 2017 to 43% in 2022), perhaps due to the revised piperacillin or tazobactam susceptibility breakpoint published in 2022. No significant change in the trend of carbapenem susceptibility has been noticed in these pathogens.2,3
In P. aeruginosa, nearly 41–44% of the isolates are found to be cephalosporin resistant.2,3 The presence of Pseudomonas-derived cephalosporinase (PDC) variants can confer resistance to cephalosporins, some variants (PDC-10, PDC-11) can hydrolyze penicillin and first-generation cephalosporins, and some can hydrolyze up to third-generation cephalosporins (PDC-2, PDC-3). In P. aeruginosa, Vietnamese extended-spectrum beta-lactamase (VEB) is identified as the most common ESBL gene, followed by TEM. More than 30% of P. aeruginosa isolates are resistant to carbapenems, and among them, NDM (41%) is predominant, followed by Verona Integron-encoded Metallo-beta-lactamase (VIM) (9%).2 Interestingly, dual carbapenemase producers of NDM with VIM or Imipenemase (IMP) is also noticed among carbapenemase-producing P. aeruginosa isolates. In the last 3 years, there has been a significant shift from VIM to IMP producers in different geographic regions of India.2,10 This change has led to NDM being the predominant carbapenemase in >95% of P. aeruginosa isolates in many hospital settings. Nearly half of the P. aeruginosa isolates are susceptible to fluroquinolones. The CLSI guideline recommends amikacin’s susceptibility breakpoints only for urinary isolates, suggesting that amikacin monotherapy may not be appropriate for treating systemic infections.11
The high carbapenem resistance rate of >80% in A. baumannii makes it challenging to treat.2,3 Nearly 55% of the isolates are susceptible to minocycline though, in the last 6 years of surveillance, there has been a drop in the susceptibility rate from 67% in 2017 to 59% in 2022.2 Among carbapenem-resistant A. baumannii, 40% of the isolates have an OXA-23-like gene and dual carbapenemase production of OXA- 23-like and NDM are identified in 60% of the isolates.2
Over 50% of infections in most ICUs in tertiary care centers in India are caused by difficult-to-treat (DTR) gram-negative pathogens. It is important to note that both ICMR and NARS-Net surveillance studies have documented dramatically higher resistance rates in isolates from the ICUs to all antibiotics in comparison to wards or outpatient clinics. In India, a sharp increase in carbapenem resistance in gram-negative pathogens is seen [Table 1], which leaves patients with limited treatment options and increases the risk of AMR-attributed mortality in patients. Further, the declining effectiveness of antibiotics imposes potentially large health and economic burdens.
Polymyxins- or tigecycline-based combinations are most often deployed as first-line therapy for treating DTR gram-negative infections. The emergence of resistance to polymyxin has already been documented in India, however, the resistance rate seems to be <10%.12 For Acinetobacter spp., neither CLSI and European Committee on Antimicrobial Susceptibility Testing (EUCAST) has defined the susceptibility breakpoints for tigecycline. Against carbapenem-resistant A. baumannii, the MIC90 of tigecycline is 8 mg/L; this elevated MIC could be due to the presence of multidrug-resistant efflux pumps.13 Minocycline retains its activity against 57% of carbapenem-resistant A. baumannii with a MIC90 of 16 mg/L.13 Despite active treatment, carbapenem-resistant A. baumannii has been reported to be associated with 40% mortality.14
Among the recent Food and Drug Administration-approved beta-lactam OR beta-lactamase inhibitors, only ceftazidime/avibactam has been approved for clinical use in India. Ceftazidime/avibactam offers broad spectrum of coverage against ESBL, ampCs, and carbapenemases such as OXA-48-like and KPCs, but lacks activity against metallo-beta-lactamase producers, including NDM.15 In Indian scenario, almost all the NDM-producing E. coli (>95%) and dual NDM/OXA-48-like-producing K. pneumoniae isolates (at least 60%) are completely resistant to ceftazidime/avibactam.16 This suggests that ceftazidime/avibactam is a reasonable alternative to standard therapy only for the treatment of infections caused exclusively by OXA-48-like-producing Enterobacterales.
The only NDM-active β-lactam treatment option available in India is the combination of ceftazidime/avibactam plus aztreonam and is viewed as a “rescue therapy” for serious NDM infections.17,18 This triple combination evades the activity of NDM enzymes as well as several other β-lactamase enzymes commonly co-produced with NDMs. More specifically, aztreonam is able to withstand hydrolysis from NDM enzymes. The β-lactamase inhibitor avibactam inactivates co-produced serine β-lactamases, enabling aztreonam to bypass hydrolysis from these enzymes and to safely reach its site of activity, penicillin-binding protein 3 (PBP3). However, susceptibility estimates of NDM-producing Enterobacterales to the combination of ceftazidime/avibactam with aztreonam are unclear, given the heterogeneity of susceptibility testing methods used for testing this combination.17 Recently, there have been reports showing the presence of novel four-amino-acid inserts (YRIN/YRIK) in E. coli PBP3, which is a worrisome phenomenon.19 PBP3 is a primary target for many beta-lactams, these four amino acid inserts reduce the affinity of PBP3 against beta-lactams such as aztreonam, ceftazidime, ceftolozane, and piperacillin.19 Moreover, PBP3 mutants in conjunction with NDM in E. coli can confer resistance to ceftazidime/avibactam with aztreonam combination.16 It is expected that in the future, cefiderocol, aztreonam/avibactam, and cefepime/taniborbactam will be available in India as NDM-targeted treatment options. Cefiderocol is a siderophore-conjugated cephalosporin with activity against NDM-producing Enterobacterales. PBP3 is also a site of action of cefiderocol, and therefore PBP3 inserts in E. coli have the ability to compromise the activity of cefiderocol. Analysis of PBP3 for the presence of 4-amino-acid insertions among E. coli isolates revealed an overwhelming proportion of isolates (97%) harbored the inserts. Cefiderocol showed limited activity against E. coli isolates cocarrying NDM with PBP3 inserts, with only 39.3% being susceptible (Dr Balaji V unpublished data). Compared to E. coli, cefiderocol exhibited an improved activity against NDM and/or OXA-48-like-producing K. pneumoniae, and 80% were susceptible (Dr Balaji V unpublished data). The activity of cefiderocol against the NDM producers was lower compared to KPC and OXA-48-like producers. The vulnerability of cefiderocol to NDM producers was likely due to a combination of the resistance mechanisms, namely, i) PBP3 insert, ii) truncated iron-binding protein, cir A, and iii) a CMY gene.20
Resistance to aztreonam/avibactam is increasingly being reported in E. coli isolates co-harboring PBP3 inserts with NDM.19 It seems that the combination of two resistance mechanisms, NDM and PBP3 inserts in E. coli, leads to a significant compromise in the activity of aztreonam/avibactam through an incremental increase in their MICs in nearly 33% of the isolates.16 Finally, the presence of the CMY-42 variant in the background of resistance mechanisms such as NDM and PBP3 inserts has been linked to elevated MICs and confers frank resistance to aztreonam/avibactam.21 This is plausible as alterations in PBP3 reduce the amount of aztreonam reaching its target, making it vulnerable to hydrolysis from CMY enzymes. Among NDM-producing K. pneumoniae, almost all isolates are highly susceptible to aztreonam/avibactam, as PBP3 insert has not been reported in K. pneumoniae.16
In the series of beta-lactamase inhibitors, taniborbactam is shown to inhibit all four Ambler class A, B, C, and D enzymes (except IMP), and its combination with cefepime has recently completed a registrational Phase 3 trial. Analysis of cefepime/taniborbactam activity based on MICs revealed excellent activity against isolates expressing OXA-48-like producers and suboptimal activity for isolates producing NDM alone or NDM with OXA-48-like. Against NDM-producing E. coli isolates, susceptibility to cefepime/taniborbactam is only 12.3%.16 On the other hand, improved activity of cefepime/taniborbactam against NDM-producing K. pneumoniae with 74.5% inhibition is observed.16 The presence of NDM with four amino-acid inserts in PBP3 of E. coli leads to a significant compromise in cefepime/taniborbactam activity.16 In K. pneumoniae, it is believed that the poor activity of cefepime/taniborbactam against such isolates could be linked with impermeability.
Cefepime/zidebactam is a beta-lactam/beta-lactam enhancer combination in Phase III clinical development. Cefepime (binds with PBP1a, 1b and PBP3) and zidebactam (binds with PBP2) have been reported to concurrently inactivate multiple PBPs, thereby triggering synergistic and pleiotropic bactericidal action that is independent of beta-lactamase inhibition. Cefepime/zidebactam potently inhibited all NDM-producing E. coli at MIC of ≤ 2 mg/L and NDM-producing K. pneumoniae at ≤8 mg/L.16 Importantly, cefepime/zidebactam readily overcomes the challenge of NDM plus PBP3 amino acid inserts in E. coli, which is attributed to zidebactam’s PBP2-binding-mediated beta-lactam-enhancer action.
Preexisting resistance mechanisms to cefiderocol, aztreonam/avibactam, and cefepime/taniborbactam even before their approval for clinical use in India are of great concern. As India has been witnessing a dominance of NDM among Enterobacterales, clinicians are desperately looking for safer and more effective substitutes for polymyxins that are currently considered salvage therapies. The aforementioned evidence indicates that cefiderocol, aztreonam/avibactam, and cefepime/taniborbactam may not be able to comprehensively address the challenge of NDMs, and there is a continued need for novel options to ensure coverage of NDM-producing Enterobacterales.
Gram-positive pathogens
In Staphylococcus aureus, > 40% of isolates are identified as MRSA [Table 2]. The incidence of MRSA is higher in ICUs (50%), compared to wards (47%) and OPD clinics (40%). There is an incremental increase in the trend of MRSA from 33% in 2017 to 44.5% in 2022.2 Erythromycin (24% vs 51%) and clindamycin (64% vs 83%) were substantially less active against MRSA than against MSSA isolates.2,3 Inducible clindamycin resistance is conferred through the acquisition of either ermA or ermC gene. In Indian S. aureus isolates, ermC (67%) was predominant while ermA (33%) was also found.22 Fluroquinolones had no activity against both MSSA and MRSA isolates. The heteroresistant vancomycin intermediate S. aureus has been reported in 12% of MRSA isolates causing bloodstream infections.23,24 Genome sequencing of hVISA isolates revealed multiple mutations in the two component systems in vraSR, graSR, and tcaRAB. 23 Vancomycin or daptomycin insusceptible S. aureus has not yet been reported in India. However, phenotypic resistance to linezolid is documented in both MRSA (2%) and MSSA (<1%) isolates, though these isolates are not studied further for the molecular resistance mechanism.2
| Resistant pathogens | % reported by ICMR | % reported by NARS-Net |
|---|---|---|
| MRSA | 44.5 | 59% |
|
Vancomycin resistant E. faecium |
27% | - |
|
Vancomycin resistant E. faecalis |
5% | - |
| VRE | - | 13% |
MRSA: Methicillin resistant staphylococcus aureus; VRE: Vancomycin resistant enterococci; NARS: National Antimicrobial Resistance Surveillance Network (NARS-Net India), ICMR: Indian Council of Medical Research.
Among Enterococcus spp., E. faecium (52%) is more common than E. faecalis (48%).2,3 Resistance to ampicillin (85% vs %24) and high-level gentamicin (64% vs 42%) is generally higher in E. faecium compared to E. faecalis.2 Similarly, resistance to vancomycin is five times higher in E. faecium (27%) than E. faecalis (5%) [Table 2]. Vancomycin resistance–encoding gene, vanA, is identified in >99% of Vancomycin resistant enterococci (VRE) isolates. In the past 6 years, the proportion of E. faecium and E. faecalis isolates resistant to vancomycin has substantially increased. In addition, resistance to linezolid is identified in 6% of E. faecium and 2% of E. faecalis isolates.2,3
Interestingly, Tn1546-like element carrying vanA gene on a novel linear plasmid has been reported.25 These linear plasmids are smaller in size compared to the circular plasmids, which facilitate rapid dissemination of vancomycin resistance in Enterococcus spp. The dual resistance mechanism of G2592T mutation in the 23S rRNA and acquisition of plasmid-meditated optrA conferring linezolid resistance have been reported in E. faecium.26 The novel plasmid (pVB3025_2) co-carrying vancomycin and linezolid-resistance determinants highlight the threat for potential dissemination.
(b) Antifungal resistance in India
Antifungal resistance is steadily increasing in India, as is being observed worldwide. Of all fungal infections, Indian ICUs report an overall incidence of invasive candidiasis at 6.51 cases/1000 ICU admissions.27,28 Among different Candida species, azole and multidrug resistance are reported in 11.8 and 1.9% isolates, respectively, with significantly higher prevalence of resistant bugs such as C. auris and C. rugosa in public sector hospitals.27,28 Resistance rates of C. auris across Indian ICUs are 58.1% against fluconazole, 13.5% against amphotericin B, and 9.5% against caspofungin, the drug of choice for this species.29 In North India, this multidrug-resistant yeast has even become the commonest species of Candida, causing candidemia in ICU settings.30 Indian studies have shown resistance in Candida species to fluconazole (3.3–64%), amphotericin B (2.1–9%), voriconazole (2.4–44%), itraconazole (1.2–69%), and echinocandins (1.7–6.2%).31 These are mostly reported in C. parapsilosis (fluconazole 32%); C. krusei (voriconazole 1.9%); C. glabrata, C. guilliermondii, C. tropicalis, and C. krusei (amphotericin B, up to 4.9%); and C. tropicalis, C. albicans, C. glabrata, and C. krusei (echinocandin, 6.2%).31,32
Among the mold infections, dermatophytes are notorious for the emergence of antifungal drug resistance in India. Resistance to terbinafine, the drug of choice for dermatophytosis, has become epidemic with development of atypical, widespread lesions and recalcitrant disease.33–36 Recurrent dermatophytosis ranges from 9–60% of the cases, with predominance of infection by T. interdigitale (66.1%) and T. rubrum (26.3%).36,37 Higher terbinafine resistance (18–61%) is noted in T. interdigitale (17–76%) and T. rubrum (17.3%).37–39 Various mutations responsible for terbinafine resistance are reported from Indian isolates, Phe397Leu, Ser395Pro/Ala448Thr, Leu335Phe/ Ala448Thr, Ser443Pro, Leu393Ser, and His440Tyr.39 Fluconazole resistance is noted at nearly 35–39.5%.37,38 No response to griseofulvin has also been noted with higher MICs to the drug.37,40 This is attributed to the rampant use of over-the-counter topical creams having steroids in addition to antifungals and antibiotics. Moreover, this has probably led to the emergence of a virulent species, T. indotineae in India, where animal reservoirs of this agent and lack of infection control are considered as challenges, which require One Health approach to tackle the situation. Higher MICs of T. mentagrophytes as compared to T. rubrum have been noticed in various Indian studies.36 The correlation between clinical resistance and higher MICs has also been noted in T. rubrum isolates.41
Invasive mold infections are reported at an incidence of 9.5 cases/1000 ICU admissions, with invasive aspergillosis and invasive mucormycosis being predominant infections.42 Azole resistance in A. fumigatus, which is quite high in Europe (>20%), is low in India (1.5–2%).43 This could possibly be due to under reporting or lower use of long-term azole therapy or non-azole fungicides. However, molecular-based study directly from respiratory samples detected azole-resistant mutations in 59% patients with chronic pulmonary aspergillosis (CPA) and 43% allergic bronchopulmonary aspergillosis (ABPA).44 Similar mutations have been reported in 7% of environmental samples in India.44 A country-wide analysis is required to determine the exact prevalence of azole resistance in Aspergillus.
GAPS AND CHALLENGES IN ADDRESSING ANTIMICROBIAL RESISTANCE
Antimicrobial resistance has established itself as one of the major global public health threats and in particular is at a grim scenario in India. In 2019, AMR was found to be directly responsible for 1.27 million global deaths and 4.95 million associated deaths. Various stakeholders have addressed AMR, however several gaps and challenges persist, which are as follows:
Overuse and misuse of antibiotics
Widespread overuse and misuse of antibiotics in human health, agriculture, and veterinary practices (driven by lack of awareness or negligence) contribute to the acceleration of AMR. In many regions, antibiotics are easily accessible without a prescription, leading to inappropriate usage.
Inadequate infection prevention and control
Weak infection prevention and control measures in healthcare facilities (due to inadequate training, infrastructure and implementation) facilitate the spread of antibiotic-resistant pathogens. Poor hygiene practices, inadequate sanitation, and insufficient access to clean water exacerbate the problem.
Limited new drug development and alternative therapies
There is a scarcity of new antimicrobial drugs in the pipeline. Pharmaceutical companies often find it economically unviable to invest in research and development for new antibiotics due to low profitability compared to chronic disease medications. The world faces an antibiotics pipeline crisis. There is inadequate research to generate a robust antibiotic pipeline in the face of rising levels of resistance, and there is also an urgent need for additional measures to ensure equitable access to new and existing vaccines, diagnostics, and medicines.
Lack of real-time and high-quality surveillance data
Many existing surveillance systems suffer from delays in data reporting and analysis, leading to a lag in identifying emerging resistance trends and implementing timely interventions. This issue is particularly pronounced in low- and middle-income countries, where robust surveillance infrastructure, standard protocols, and data-sharing capabilities are lacking.
Global coordination
AMR is a transnational issue requiring global cooperation, however, coordination among countries is often lacking. Fragmentation in policies, regulations, and standards hinders collective action against AMR. The Global Antimicrobial Resistance Surveillance System (GLASS) study aimed to standardize data collection, analysis, and monitoring of AMR on a global scale. However, it highlighted the stark scarcity of data from resource-limited settings. Constraints such as limited laboratory capacity, inadequate infrastructure, and funding shortages severely hamper their ability to effectively collect, analyze, and report AMR data. Furthermore, funds to fight AMR are not available proportionate to the severity of AMR problem.
Lack of public awareness
Low awareness among the general public, healthcare providers, and policymakers about the seriousness of AMR and the actions needed to mitigate it remains a challenge. Effective communication strategies are needed to increase awareness and promote responsible antimicrobial use. More importantly, AMR has not found its due weightage in political discussions.
Lack of trained manpower
The shortage of skilled personnel undermines the effectiveness of surveillance efforts and hampers the ability to detect, monitor, and respond to emerging threats of antimicrobial resistance. Insufficient access to training programs and continuing education opportunities for healthcare professionals in resource-limited settings limits their capacity to conduct AMR surveillance activities. Without adequately trained professionals, there is a risk of inaccurate data collection, suboptimal laboratory testing, and inadequate analysis and interpretation of surveillance data.
Lack of well-equipped laboratories
Many lower and middle income countries (LMICs) lack well-equipped laboratories with trained personnel capable of conducting accurate and comprehensive AMR testing. This leads to incomplete or unreliable data on AMR patterns and trends, hindering the ability to effectively monitor and respond to antimicrobial resistance.
Lack of preparedness for outbreaks
Inadequate planning and resources devoted to AMR surveillance limit the ability to detect and respond to outbreaks of antimicrobial-resistant infections effectively. Without robust surveillance systems in place, there is a risk of delayed detection and response to emerging AMR threats, leading to increased morbidity, mortality, and healthcare costs.
Nonavailability of antifungal resistance testing
Access to reliable and standardized antifungal susceptibility testing methods remains limited. The emergence of multidrug-resistant Candida auris, terbinafine-resistant dermatophytes, and azole-resistant Aspergillus fumigatus highlights significant challenges in fungal infection management. These challenges require concerted efforts to raise awareness about fungal infections, improve training and education in mycology, and expand access to diagnostic mycology services.
Economic impacts
In addition to death and disability, AMR has significant economic costs. The World Bank estimates that AMR could result in US$ 1 trillion additional healthcare costs by 2050, and US$ 1 trillion to US$ 3.4 trillion gross domestic product (GDP) losses per year by 2030. Governments should step up to compensate the economic the economic impacts of AMR.
Addressing AMR requires a coordinated effort involving governments, healthcare professionals, researchers, pharmaceutical companies, and the public. Multisectorial collaboration, sustained investments in research and development, strengthened surveillance systems, and robust AMSP are essential for combating AMR and preserving the effectiveness of available antimicrobial drugs for future generations.
Lack of livestock AMR surveillance data
There is concerningly limited data on AMR’s quantitative impact on current livestock production, which is mainly due to a lack of AMR surveillance and comprehensive data collection. The intergovernmental public health systems that track cases of resistance in humans offer little data on AMR in livestock, leaving a glaring gap in knowledge. Most imperatively, livestock AMR data deposits do not identify how these hotspots translate to financial and production loss; so, we are likely significantly underestimating AMR’s current and future consequences. Without a true understanding of the present impact of AMR on animals, efforts to establish effective preventative measures to mitigate future impacts are stifled.
Implementation of NAP-AMR
Implementation remains fragmented and siloed, and greater political commitment and investment is needed. Marked gaps and variability in maturity of NAP development and operationalization across the domains of: i) policy and strategic planning; ii) medicines management and prescribing systems; iii) technology for optimized antimicrobial prescribing; iv) context, culture, and behaviors; v) operational delivery and monitoring; and vi) patient and public engagement and involvement; and vii) violations in the existing government laws and enforcements were seen. Further, there is a lack of financial allocation across states, and poor enforcement and inadequate multisectoral coordination have hampered progress. Implementation and enforcement of Schedule H1 have lagged far behind and have not resulted in reductions of nonprescription, over the counter (OTC) antibiotic use owing to poor regulatory enforcement by drug inspectors as well as limited capacity.
The gaps, actionability and impact have been summarized in the Table 2a below.
| Gaps | Actionability | Impact | |
|---|---|---|---|
| Institutional | Noninstitutional | ||
| Overuse/misuse of antibiotics | +++ | + | +++ |
| Inadequate infection prevention & control (IPC) | +++ | + | +++ |
| Lack of stewardship (diagnosis, therapy) | +++ | + | +++ |
| Lack of rapid diagnostics | ++ | ++ | ++ |
| Education | ++ | + | +++ |
| Lack of trained manpower | +++ | ++ | +++ |
| Lack of well-equipped laboratories | +++ | ++ | +++ |
| Delay in availability of newer antibiotics | ++ | + | +++ |
| Lack of real-time quality surveillance | ++ | + | ++ |
| Lack of public awareness | + | + | ++ |
| Economic impacts | +++ | + | + |
| Global coordination | ++ | ||
| Lack of preparedness for outbreaks | - | - | + |
| New drug development | - | - | + |
INTERVENTION STRATEGIES FOR CONTROL OF ANTIMICROBIAL RESISTANCE
1. General recommendation
Escalating resistance to antibiotics, including the most potent and last-line agents, is an urgent threat to global public health. The roots of AMR are multifactorial [Table 3]; the emergence of resistant strains has quickly followed the introduction of almost every new antibiotic, beginning with penicillin and continuing to the newest additions, ceftazidime/avibactam, ceftolozane/tazobactam, imipenem/relebactam, meropenem/vaborbactam, and cefiderocol.45 Arguably, the largest contributor to AMR is the increase in antibiotic prescribing. From 2010 to 2015, global consumption of antibiotics increased by 65%.46 In addition, climate change is expected to increase water- and vector-borne febrile illnesses, resulting in increased antibiotic exposure from empirical therapy. There are numerous opportunities for straightforward interventions formulated for the containment of AMR [Table 4].47 Interventions to deal with AMR, from simple actions to complex ones, from regulatory to behavioral approaches, and from strategies focusing on infection prevention to those focusing on responsible use of antimicrobials, are crucial to consolidate an evidence-based approach to the challenge.48,49
Microbial factors
|
Antimicrobial factors
|
Human factors
|
Diagnostics and surveillance
|
Minimize overdiagnosis
|
Minimize overuse
|
Alternative
|
2. Interventions for policymakers
Expedited implementation of national action plan on antimicrobial resistance (NAP-AMR) 2.0
The Government of India has formulated a national action plan (NAP) to tackle AMR (NAP- AMR), largely modeled on the World Health Organization (WHO) global action plan on AMR [Figure 1].50 The 5-year NAP-AMR has been established in three states or union territories, namely Kerala, Madhya Pradesh, and Delhi;51 financial constraints have also impeded further implementation efforts. The six strategic priorities of the NAP-AMR include:
-
Improving awareness and understanding of AMR through effective communication, education, and training
-
Strengthening knowledge and evidence through surveillance
-
Reducing the incidence of infection through effective infection prevention and control
-
Optimizing the use of antimicrobial agents in health, animals, and food
-
Promoting investments for AMR activities, research, and innovations, and strengthening India’s international, national, and state-level collaboration and leadership on AMR
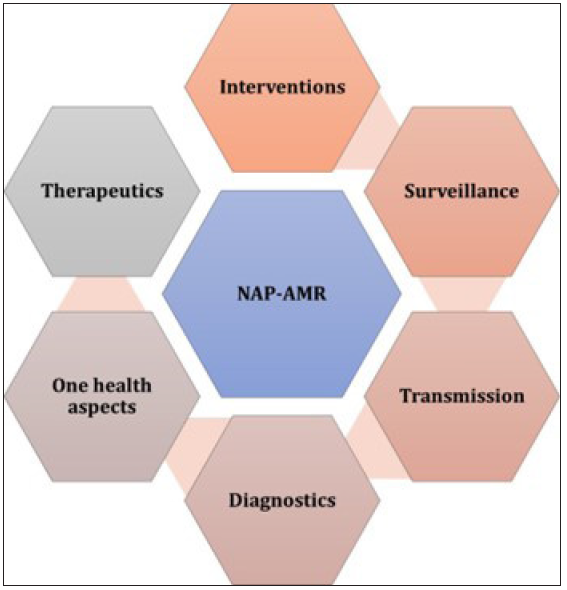
- National action plan for the containment of antimicrobial resistance (NAP-AMR).
First, the plan relies heavily on individual knowledge, attitudes, and practice (KAP) surveys across the general population and on behavioral studies. Second, the indicators in the plan rely heavily on training, guidelines, and behavior change interventions with prescribers (doctors, nurses, pharmacists, among others) that have seen limited success in India. Though, the plan mentions the need for antimicrobial stewardship at different levels, it neither recognizes the diversity of outpatient care provision in India nor provides clear mechanisms to coordinate activities between the public and private sectors. For AMR surveillance, the NAP-AMR relies on a national network of laboratory-based surveillance at a few designated reference laboratories in tertiary care medical institutions.
ICMR has taken the initiative to address this gap by establishing antimicrobial resistance surveillance and research network (AMRSN) and implementing AMS programs in tertiary care hospitals.52 The data collected through these initiatives do not adequately reflect the comprehensive picture of AMR in the country, particularly from community healthcare settings. Limited standardized surveillance data make it even more challenging to monitor the extent and scope of AMR, and most of the data come from published studies of HAIs in inpatient settings, scoping reports, prospective studies, and point prevalence surveys at select, large hospitals. However, antibiotics are routinely prescribed for respiratory infections in primary care and outpatient settings both in the public and private sectors. It is also important to note that secondary care hospitals in India are not well equipped to document the patterns of local antibiograms and monitor antibiotic usage due to the absence of good clinical microbiology labs and skilled staff. From a clinician’s perspective in India, recent local susceptibility data would assist in the selection of empirical antibiotics for community-acquired infection management and to support rational choices when treating these bacterial infections. However, India-specific community-acquired pathogen AMR surveillance is lacking.53 Therefore, public education is vital, covering the misuse of antibiotics, such as not purchasing OTC antibiotics and also the need to complete the full course as prescribed by the physician.
ICMR has identified six nodal centers or each pathogenic group where the organisms with unusual resistance can be confirmed. This includes All India Institute of Medical Sciences, New Delhi, for typhoid fever; Christian Medical College and Hospital, Vellore, for non-fermenting gram-negative pathogens and diarrheal pathogens; Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry, for gram-positive pathogens, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, for Enterobacterales and fungal pathogens; and the National Institute of Cholera and Enteric Diseases (NICED), Kolkata, for cholera and diarrheagenic pathogens.
Absolutely, the dynamic nature of AMR demands a flexible and a responsive approach to combat it effectively. Viewing the framework as a cyclical process allows for ongoing assessment, adaptation, and improvement in response to the evolving challenges posed by AMR [Figure 2]. This iterative approach enables policymakers, healthcare professionals, and other stakeholders to continually refine and update NAPs on AMR in line with emerging evidence, changing patterns of resistance, and shifts in healthcare practices. By embracing this cyclical process, it would be helpful to address the multifaceted aspects of AMR and enhance the effectiveness of their strategies to preserve the efficacy of antimicrobial agents.
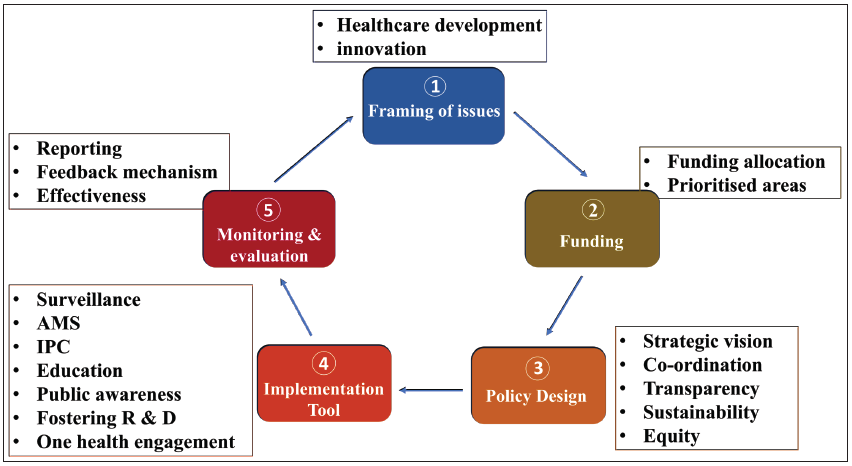
- An adapted conceptual framework for assessment of NAP-AMR. AMS: Antimicrobial stewardship, IPC: Infection prevention control, R & D: Research and diagnostics, NAP-AMR: National action plan on antimicrobial resistance.
Recommendations for action
-
Extend the current AMRSN to all tertiary care hospitals in the country, with at least one from each state.
-
Start a separate AMRSN network or subcategory for secondary-level or district hospitals to better reflect resistance patterns at the community level.
-
Start an AMRSN exclusively for community pathogens from outpatients with representation from each state (e.g., Streptococcus pneumoniae, Neisseria gonorrheae, community-acquired methicillin resistant staphylococcus aureus (CA-MRSA), Salmonella typhi and S. paratyphi).
Multisectoral engagement
The multifaceted complexities of AMR require consistent action, a multidisciplinary approach, and long-term political commitment. Multisectoral collaboration is the deliberate coordination of different stakeholder groups—such as government, civil society, the private sector, and sectors such as health, agriculture, trade, and education—and the environment to jointly achieve coordinated and effective action on AMR [Figure 3]. It includes horizontal collaboration across sectors and vertical collaboration across levels. Vertical collaboration, from local to global levels across sectors, and from on-the-ground practitioners to central policymakers within individual sectors, can be achieved through both top-down and bottom-up approaches. Horizontal collaboration across different government departments and nongovernment stakeholders, can be supported through diverse activities, including knowledge-sharing platforms and multistakeholder forums. A formal, resourced administrative structure at a level above the implementing ministries is generally required for strategic direction and oversight, however, in practice, there is no one-size-fits-all approach to AMR governance. In the long term, effective multisectoral collaboration requires governments to take ownership of the NAP implementation process and ensure it is appropriately resourced and given sufficient visibility to keep it a national priority.
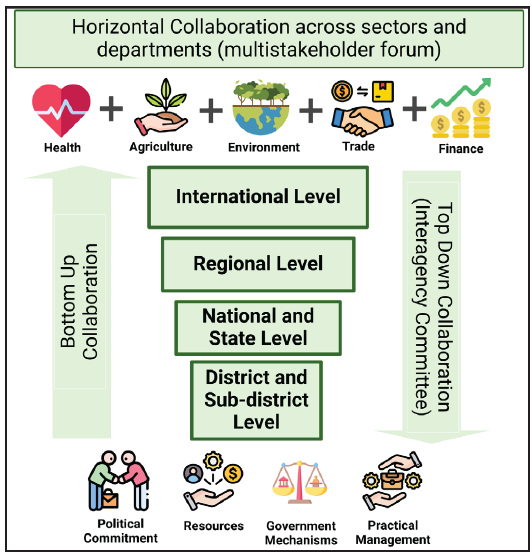
- Multisectoral collaboration can be both horizontal across sectors as well as vertical across levels to contain antimicrobial resistance (AMR).
In a tiered approach, different levels have different functions. At the top lies a high-level, multisectoral, decision-making body that sets the strategic direction and allocates resources. There should also be coordination at an operational level where those implementing interventions come together to ensure coherence. There should be operational-level, active, discrete units within ministries, civil society, and private sector partners responsible for implementing the activities listed in the NAP. Experience at the country level indicates that it is best to keep the top level relatively small, to prevent it from becoming unwieldy and unworkable. One option for keeping the top-level body lean and efficient is to ensure that its members are very well-connected and that they can effectively represent multiple stakeholder groups.
An “AMR champion” authority to work across sectors that galvanizes the interest of high-level policymakers at ICMR, NCDC, and department of biotechnology (DBT) triggers effective action to tackle AMR. Consistent engagement and information sharing between stakeholders working in the AMR space using available government platforms was critical in engaging and sustaining government leadership and commitment.
Recommendation for actions
-
Identify and use champions and events to raise the profile of AMR and sustain its visibility on the political agenda
-
Use local data on AMR to illustrate likely local and national impacts and convince key decision-makers of the need for action
-
Use government platforms to share and promote AMR action
-
Manage the risks of changes in leadership to ensure that AMR remains on the political agenda
-
Support nongovernment multistakeholder working groups and forums to share information and resources
-
Adopt a participatory approach that engages nongovernment stakeholders to develop a shared vision and commitment to tackle AMR
-
Understand that AMR initiatives can build on existing programs and activities
-
Leverage existing policies and plans to mainstream AMR and optimize resources
-
Establish a clear system or structure for coordinating AMR action across all relevant national plans
Funding allocation
Currently, there is inadequate financial support available for the sustainable implementation of NAPs. Increased investment is urgently needed to support the delivery of NAPs. More financial support and incentives are required for effective and affordable innovations across all sectors and stakeholders (including the private sector) to secure a sustainable pipeline for new antimicrobials (particularly antibiotics), vaccines, diagnostics, waste management tools, and safe and effective alternatives to antimicrobials, and to ensure equitable access to them. The antimicrobial resistance multipartner trust fund (MPTF) combats the threat of antimicrobial drug resistance through strategic collaboration, sustainable streams of capital, and sustainable development goal-focused responses that support localized “One Health” NAPs. The studies by the Global AMR research and diagnostics (R&D) Hub to evaluate the scale of challenge of bringing the needed new antibiotics (and diagnostics) into the market in current economic conditions highlighted the astonishing mismatch between global patient needs and the commercial potential of products. The immediate adaptation of existing national health systems tools in combination with pull incentives was called for to support innovation and to ensure that the necessary new products are accessible to those with the greatest need around the world. The Biomedical Advanced Research and Development Authority (BARDA), since its launch in 2011, has provided $1.5 billion in funding. As of June 2021, BARDA’s portfolio includes 16 antibacterial programs to address drug-resistant bacteria that the Centre for Disease Control and the WHO consider serious global threats.
Promoting vaccination to minimize infections
Preventing infections using vaccination reduces antibiotic use, which is one of the main drivers of AMR. There are vaccines available against three priority bacterial pathogens: pneumococcal disease (Streptococcus pneumoniae), Haemophilus influenzae type b (Hib), and Typhoid fever (Salmonella typhi).
Unfortunately, most vaccines developed against the main resistant pathogens are still under preclinical and clinical evaluation due to the complexity of pathogens and technical difficulties. Vaccines against these pathogens are unlikely to be available in the short term, and alternative interventions should be pursued urgently to prevent resistant infections due to priority bacterial pathogens. Vaccination also reduces carriage (colonization of an individual in the absence of disease) and shedding bacteria, thus limiting the spread of infections within a community (herd protection) [Figure 4]. It is vital to support the uptake of licensed vaccines by implementing the below strategies:
-
Enhance public awareness of the importance of vaccination in the fight against AMR to improve vaccine confidence, uptake, and coverage
-
Improve human vaccination coverage in all age groups, with an emphasis of including adult immunization in national immunization plans (NIP) and implementing targeted vaccination of at-risk populations
-
Align AMR NAPs with NIPs, and setting clear and defined vaccine uptake targets
-
To develop surveillance systems to monitor the impact of both new and established vaccines on AMR
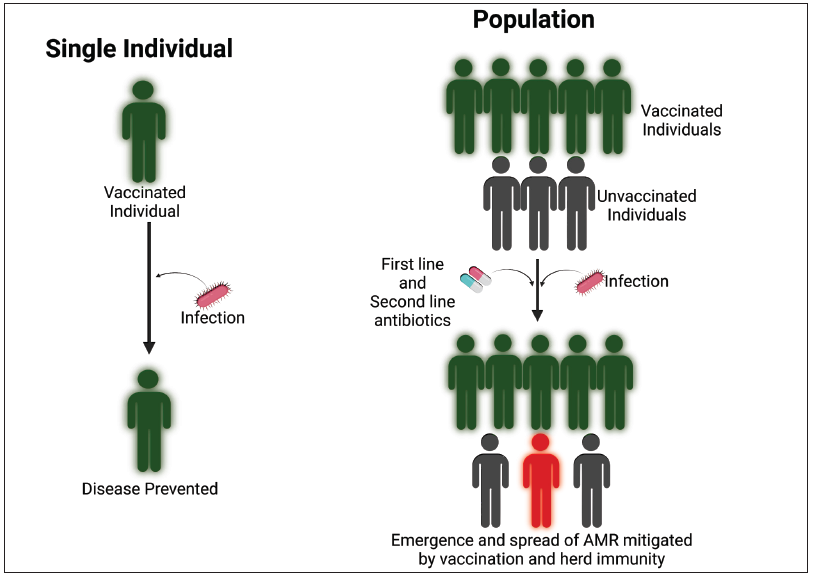
- Vaccines against antimicrobial-resistant pathogens could prevent or reduce life-threatening diseases and thus decrease health care costs, and also reduce the use of antibiotics (both first-line and second line drugs) with the potential of decreasing the emergence of AMR. If sufficient vaccine coverage is achieved in a population, indirect protection (herd immunity) further prevents spread of resistant strains. Decreased disease burden would also negate the need for antibiotics.
For instance, the introduction of a 13-valent conjugate pneumococcal vaccine has shown the impact of vaccination on the incidence of pneumococcal disease. Antibiotic-resistant invasive pneumococcal disease declined in both vaccinated and unvaccinated populations.54 The effectiveness in reducing AMR has also been proven by vaccination against Hib. Before the introduction of the Hib conjugate vaccines, 16.6% of all Hib strains worldwide were beta-lactamase positive which reduced the treatment options drastically. With the routine use of Hib conjugate vaccines, disease cases have dropped significantly together with the number of beta-lactamase-positive strains.55 Influenza vaccination has been demonstrated to reduce the use of antibiotics by 64% in vaccinated individuals by reducing the incidence of disease and thereby reducing the number of associated antimicrobial prescriptions.56 Notably, during the pandemic, antibiotics were used in 75% of the patients suffering from severe COVID-19, while only 15% of those patients actually developed bacterial superinfections.57 There are 11 vaccines aiming to address AMR in the vaccine pipelines, targeting six high-threat microorganisms, at different stages of clinical development [Table 5].58
| Microorganism | Number of vaccines in the pipeline | Status | Trial population | Technology Platform |
|---|---|---|---|---|
| C. difficile | 3 | Phase I, II, III | Adults |
Toxoid vaccine Protein subunit |
| E. coli (ExPEC) | 1 | Phase III | Adults | Glycoconjugate vaccine |
| K. pneumoniae | 1 | Phase I | Adults | Glycoconjugate vaccine |
| Shigella spp. | 1 | Phase II |
Paediatric, Adults |
Glycoconjugate vaccine |
| S. aureus | 1 | Phase II | Adults | Glycoconjugate vaccine |
The action framework describes a vision for vaccines to contribute fully, sustainably, and equitably to the prevention and control of AMR and identifies a series of priority actions to be taken by different stakeholders in the fields of immunization and AMR.59 It focuses on three areas:
-
Expanding the use of licensed vaccines to maximize impact on AMR
-
Developing new vaccines that contribute to the prevention and control of AMR
-
Expanding and sharing knowledge on the impact of vaccines on AMR
Recommendations for action:
-
Promoting the NIP and improving implementation to reach close to 100% of the childhood population.
-
Adding to the NIP vaccines that are efficacious but not currently part of the NIP or not uniformly used across the country (e.g., conjugated pneumococcal and conjugated typhoid vaccines)
-
Initiating a National Adult Immunization Program for high risk individuals (age > 65, younger persons with comorbidities), starting with influenza and pneumococcal vaccines.
Mandatory notification of priority pathogens
The multidrug-resistant (MDR) pathogens should be listed and considered for mandatory notification (isolate needs to be sent to and confirmed as resistant at reference lab). Recently, in 2024, the WHO has revised the priority pathogen list, for which new antibiotics are urgently needed. This includes the critical group (carbapenem-resistant A. baumannii), carbapenem-resistant Enterobacterales, third-generation cephalosporin-resistant Enterobacterales), high priority group (fluroquinolone-resistant S. typhi, fluroquinolone-resistant Shigella spp., fluroquinolone-resistant non-typhopidal Salmonella, carbapenem-resistant P. aeruginosa, third-generation and/or fluroquinolone-resistant N. gonorrhoeae, MRSA), and medium priority group (Group A Streptococci, macrolide-resistant S. pneumoniae, ampicillin-resistant H. influenzae, and penicillin-resistant Group B Streptococci). Sending alerts mainly focusing on the critical and high priority group of pathogens would be helpful for infection control and to promote antimicrobial stewardship (ASP) practices.
-
Carbapenem- and colistin-resistant E. coli, K. pneumoniae, A. baumannii, and P. aeruginosa
-
E. coli with presumed PBP insert showing resistance to the triple combination of ceftazidime–avibactam plus aztreonam
-
Vancomycin nonsusceptible or resistant Staphylococcus aureus
-
Penicillin- and cephalosporin-resistant Streptococcus pneumoniae using cerebrospinal fluid breakpoints
-
Third-generation cephalosporins and azithromycin-resistant Salmonella typhi and S. paratyphi
-
Cephalosporin- and fluoroquinolone-resistant Neisseria gonorrheae
-
Penicillin-resistant Neisseria meningitidis
-
Echinocandin-resistant Candida auris
-
Aspergillus species resistant to voriconazole
Recommendation for action:
-
Reporting of the above-mentioned pathogens should be made mandatory on the part of the center from where it is isolated (after confirmation by a reference laboratory).
-
Data on the incidence of these MDR pathogens, including spatial and temporal distribution, should be available on a national public website such as NCDC. Spatial and temporal distribution of pathogens provide evidences for public health emergency preparedness.
Public health interventions to improve AMR awareness
A large proportion of AMR infection is linked to community-associated infections, suggesting that interventions set in community settings, including primary care, are urgently needed. When considering the community setting, emergence and amplification of AMR are driven by numerous factors such as the nonprudent use of antimicrobials, lack of access to clean water and poor sanitation, and limited access to quality therapeutics, vaccines, or diagnostics.59–61 Each of these AMR determinants has unique intricacies that require targeted interventions.
The Indian government has introduced “Red Line campaign” to improve awareness among the public and healthcare professionals about the importance of appropriate use of antibiotics.61,62 In this initiative, antibiotics and certain other prescription-only medicines had a bold red-colored line on the blister pack to indicate that these drugs were to be consumed only on the advice of qualified prescribers. However, this initiative limited success in regulating OTC antibiotic sales and in creating awareness about antibiotic misuse and overuse in the community.63 A study conducted in India showed that only 7% of healthcare professionals could describe the significance of the red line campaign and none among patients.63 General practitioners (GPs) play an essential role in national efforts to tackle AMR, as they prescribe the largest volume of antibiotics. By following best practice prescribing, they can reduce the amount of antibiotics used and contribute to decreasing antibiotic resistance. Consequently, there is a need for educational activities among GPs to improve the rational use of antibiotics, building on current and planned activities by the Ministry of Health.
Patients presenting in primary care with respiratory, urinary, skin, or dental infections account for the majority of antibiotic prescriptions. It is, therefore, important that primary care physicians be kept well-informed of the AMR landscape and be conversant about the important measures by the government systems in controlling AMR. Also, it is important to strengthen antimicrobial stewardship strategies in the community by implementing and evaluating community interventions to tackle AMR. There have been many interventions targeted at clinicians, patients, and the public [Table 6]. The nonprudent or nonprescribed use of antibiotics is linked to knowledge, attitudes, and practices that may determine inappropriate prescribing, self-medication, and antibiotic use without prescription. Many of these are clearly linked to human behavior, which calls for a need to understand the types of efficient interventions. Clinicians should play an active role in the education of patients, informing of the risks of the acquisition of resistant bacteria.
| ‐ | Intervention | Target |
|---|---|---|
| During the consultation |
|
Clinician-focused |
|
Clinician and patient- focused | |
| Outside the consultation | National antibiotic awareness campaigns | Public |
Public education campaigns have shown to be effective in changing attitudes and knowledge regarding antibiotic use and resistance.64,65 Clinicians should support the education of patients regarding antibiotic use and resistance. They could use effective strategies, such as shared decision-making, to alert people of the actual risk of acquiring antibiotic-resistant bacteria following antibiotic use. Patients need to stay informed and receive independent information on antibiotics, as better health literacy and a higher degree of knowledge and awareness about the appropriate use of antibiotics are associated with decreased consumption. Social media for health intervention has been widely used during the COVID-19 pandemic, which was found to be the fastest mode of communication for the distribution of preventive information and it could be efficiently be used for education, knowledge dissemination, and healthcare awareness.66 However, social media in AMR public health interventions is not well explored and potentially underused. Intervention showing animated films and musical or theatre shows had a positive impact on the knowledge gained and attitudes of the participants.
Given the diagnostic challenges in outpatient settings, scaling-up use and access of point-of-care tests, such as C-reactive protein and procalcitonin, may reduce unnecessary antibiotic use.67 Improving microbiology support, continuous surveillance of antimicrobial resistance patterns, implementation of antibiotic policy at all levels of healthcare, continuous awareness generation among medical students regarding rational use of antibiotics, and regular prescription audits are some of the other widely recommended measures, all of which are seriously lacking in India.68
Recommendations for action
-
A national education campaign for the public on the dangers of self-medication of antibiotics or their use without a prescription should be introduced in mainstream media, for example, newspapers and television as well as social media (e.g., similar to current campaigns to discourage tobacco)
-
Ensure clean drinking water and improve sanitation and personal hygiene (including hand hygiene)
-
Develop mechanisms to return unused antimicrobials from households for safe disposal
-
Raise awareness of the role of vaccines in limiting the emergence of AMR and use of antibiotics
-
Ensure the availability and affordability of preventative testing and counseling services for common infections
Promoting curriculum learning on antimicrobial resistance
Among health workers, a variety of factors can result in the misuse or overuse of antimicrobials, including a lack of knowledge or up-to-date information, inability to identify the type of infection, yielding to patient pressure to prescribe antibiotics, and a preponderance of situations that allow for financial benefit from the supply of antibiotics.69 Therefore, AMR education and training resources are crucial to support educators, decision-makers, and health policy planners in implementing effective policies to guide actions on AMR control. Implementation of AMR competency framework, which is matrix of the AMR domains, and health worker categories and their competencies (the knowledge, skills and attitudes) is necessary to effectively address AMR in practice settings [Table 7].70 The framework adopts an interprofessional approach based on the principle that addressing AMR requires a shared understanding and an effective collaboration and communication among health workers. Given that a number of different health workers are involved in the sequence of events and scenarios leading to the prescription and use of antimicrobials, the categorization of health workers has been structured to reflect, in a comprehensive manner, the most significant roles impacting antimicrobial prescription and use. In India, the introduction of AMR education in pharmacy curricula showed potential benefit to take key role in antimicrobial stewardship, where postgraduate training for pharmacists remains limited.71 The following teaching methods or training techniques can be used to deliver the curricula depending on the learning objective, audience type, learning environment, and availability of technology.72
-
Interactive lectures
-
Interactive, small, group tutorials using problem-solving exercises and case-based learning, which encourage the trainee to present, analyze, and discuss
-
By apprenticeship, learning by doing (as in in-service training and practical laboratory-based exercises)
-
Role playing for preservice and in-service education
-
Using e-learning modules such as massive open online courses and webinars
-
Project-based learning with creation of project reports, strategic papers, and critical appraisal of the literature
| Antimicrobial resistance domainsa | Category 1: All health workersb | Category 2: Prescribersc | Category 3: Non-prescribersd |
Category 4: Public health ofAicers/ health services managerse |
||
|---|---|---|---|---|---|---|
| Nurses | Pharmacists | Laboratory scientists/ technicians | ||||
|
Foundations that build awareness of antimicrobial resistance Competency statement: Health worker demonstrates that they have the knowledge and awareness of effective approaches to control AMR, and has the skills/attitudes to implement change according to role and level of training |
Relevance: HighKnowledge:
|
Relevance: HighKnowledge:
|
Relevance: High Knowledge:
|
Relevance: High Knowledge:
|
Relevance: High Knowledge:
|
Relevance: High Knowledge:
|
Skills:
|
Skills:
|
Skills:
|
Skills:
|
Skills:
|
Skills:
|
|
Attitudes:
|
Attitudes:
|
Attitudes:
|
Attitudes:
|
Attitudes:
|
Attitudes:
|
|
AMR: Antimicrobial resistance
Recommendations for action
-
Antimicrobial resistance and strategies to address it should be included in the core curriculum at the MBBS level, preferably at the time Microbiology and Pharmacology subjects are taught.
-
Re-education of postgraduates on antibiotic prescribing etiquette should be included in the curriculum to sustain the knowledge, perception, and attitude toward antibiotic resistance as well as proper antibiotic use and prescription. The re-education may be made mandatory during reregistration of doctors.
-
Infectious Diseases and Microbiology departments need to be started in each state at all tertiary care hospitals and hospitals that conduct postgraduate training. AMR should be made part of the core curriculum for postgraduates in Medicine and Allied specialties and Microbiology.
-
A minimum number of credit hours of continuing medical education on AMR should be introduced for the re-licensure of practicing physicians.
-
A modified Miller’s pyramid [Figure 5] can be applied to rate the level of achievement of each individual learning point
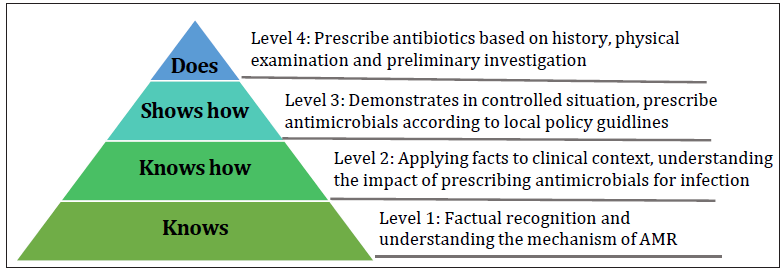
- Modified Miller’s pyramid to rate level of perceived output for each learning points. AMR: Antimicrobial resistance.
3. Intervention strategies at regulatory level
Banning irrational fixed-dose combinations
Fixed-dose combination (FDC) definition as per WHO states that, a combination of at least two active ingredients in a fixed proportion of ratio. The FDCs are justified when they demonstrate clear benefits in terms of (a) potentiating the therapeutic efficacy; (b) reducing the incidence of an adverse effect of drugs; (c) having pharmacokinetic advantage; (d) better compliance by reducing the pill burden; (e) reducing the dose of individual drugs; and (f) decreasing the development of resistance. An FDC is described as irrational if these conditions are not met. As per the Rule 122E of the Drugs and Cosmetics Act 1940, the FDCs are considered as new drugs and the Central Drugs Standard Control Organization (CDSCO), after due examination of data on rationality, safety, and efficacy issues approval. Based on this, the State Licensing Authority (SLA) gives the manufacturing and marketing permission. Incidentally, in the past, SLAs issued the license to manufacture and market without asking for non-objection from CDSCO. Thus, the efficacy, safety, and rationality of such FDCs remain questionable. This “disconnect” between the CDSCO and SLAs has precipitated a roadblock in the action against irrational FDCs. The CDSCO, headed by the Drugs Controller General of India, sought endorsement from infectious diseases physicians, microbiologists, and pharmacologists before FDC became available in the market. The Kokate Committee was constituted by the Ministry of Health and Family Welfare, Government of India, to critically assess the safety and efficacy for the approval of FDCs as rational or irrational.
Manufacturing and marketing of many FDCs play a crucial role in escalating AMR. India has a federal system of government, with drug regulatory functions divided between central and regional authorities.73 In particular, the WHO does not recommend the use of FDC in clinical practice.74 Regional authorities (states and union territories) grant licenses for manufacturing, selling, and distributing drugs. Before manufacturing, licenses for new drugs can be granted, however, manufacturers must obtain prior approval from the central regulatory authority, the Central Drugs Standard Control Organization headed by the Drugs Controller General of India (DCGI), for a period of 4 years. FDCs are a hallmark of the Indian drug market. It is estimated that 68% of FDCs containing antimicrobials on the Indian market have not been approved by the central drug regulator.75 During 2011–2012, the sale of FDC antibiotics in India consisted of 499 million units containing key access antibiotics, 367 million units containing watch group antibiotics, 3 million units containing reserve group antibiotics, and 3 million units containing uncategorized antibiotics.76 The central government has responded with several measures to control unapproved FDCs, which include the prohibition of manufacturing, distribution, and sales of some FDCs. In 2016, the government issued an official notification banning many FDCs that had been licensed for the manufacturer without prior approval from DCGI, following the recommendations of an ad hoc technical assessment of the Kokate Committee set up by the Ministry of Health and Family Welfare.77–80
Recommendations for action:
-
A list of irrational FDCs based on the WHO recommendations should be drawn up, and all such FDCs should be banned from the market.
-
Both the central and state regulators must harmonize their procedures for licensing FDCs, and the enforcement mechanism needs to be strengthened
-
No future antibiotic FDC should be licensed without approval from an appropriate central expert committee
Restricting over-the-counter sales of antibiotics
Over-the-counter (OTC) sales of antibiotics is a common practice. Nearly 52% of Indians were estimated to self-medicate themselves, due to lack of time and to avoid doctors’ fees.81,82 Both access and watch groups of antibiotics are often dispensed for viral and self-limiting conditions, including fever, cold, cough, and sore throat.83 In India, the sale of antibiotics is regulated by introducing Schedule H1 under the Drugs and Cosmetics rules 1945, to mitigate AMR.84 This initiative tightens the restrictions on the sales of prescription-only medicines, listed in and covered by Schedule H1 of the Drugs and Cosmetics Rules. Currently, 46 drugs have been placed under this restricted category, which mainly comprises third- and fourth-generation cephalosporins, carbapenems, newer fluoroquinolones and first- and second-line antitubercular drugs. The packaging of these drugs will have a mandatory Schedule H1 warning printed on the label in a box with a red border and the Rx symbol in red. For Schedule H1 drugs, pharmacists are required to maintain a separate register for their sales and retain prescription copies.74 This act is implemented mainly to avoid OTC sales of antibiotics without a valid prescription or to prevent pharmacists from dispensing antibiotics on their advice to the patient. However, initiatives led by the government have found limited success in regulating OTC antibiotic sales.
Financial and resource constraints may have contributed to inadequate awareness and suboptimal implementation of regulations planned by the government. Alternatively, in the community, OTC sales can be limited by raising the bar for any antibiotic to a higher schedule, the current Schedule H1 included only a few drugs and also with poor implementation. For example, the complete banning of sedatives in OTC that makes it difficult to access.
To address issue of OTC sale of antibiotics, multifaceted strategies are needed, extending beyond administrative or regulatory measures. Moreover, dispensing practices should be better regulated, at least limiting the use of antibiotics to those belonging to the access group. We believe there is an urgent need to foster a proactive attitude among pharmacists through a combination of educational interventions within the community pharmacy sector and increased awareness campaigns targeting proper antimicrobial use among the general population.
Recommendations for action:
-
All antibiotics in the WHO watch and restricted categories need to be placed in schedule H1 and should be dispensed strictly with a doctor’s prescription only. Safeguards to prevent inappropriate or OTC use that currently exist for sedatives and narcotics need to be extended to all drugs in schedule H1.
Fast-track approval for new antibiotics
In India, many patients lack access to newer antibiotics and are challenged by poor economic incentives, regulatory hurdles, and poor health infrastructure. There was a significant lag between India and other developed nations in accessing the Food and Drug Administration, United States-approved new antibiotics.85 Fast Track speeds the development and review of new antibiotics by increasing the level of communication between DCGI and drug developers [Figure 6]. In 2019, to fulfill the objective of fast-tracking the accessibility of new drugs and promoting clinical research in India, the Union Ministry of Health and Family Welfare, India has notified the “New Drugs and Clinical Trials Rules.”86 Phase III of clinical studies can be overlooked in India, if the drugs are already approved in other countries. Clinical studies can only be exempted if there is no serious adverse effect reported for the approved molecule, and there should not be any significant differences in the metabolism pathway in the Indian population. This decision has led to easier access of pharmaceuticals to the Indian population and, hence improving the health status of Indians.85,86
- Traditional vs. accelerated development and approval process for novel antibiotics.
Recommendations for action:
-
Existing drugs approved abroad need to be given unrestricted accelerated approval in India without further studies (e.g., cefiderocol, sulbactam–durlobactam).
-
Drugs not approved abroad that are promising in treating MDR pathogens, such as cefepime–zidebactam, need compassionate or emergency use authorization and pathways to accelerated approval.
-
Develop successful approaches to making such antibiotics available as rapidly as possible, through breakthrough therapy (expedite the development and review of drugs which may demonstrate substantial improvement over available therapy); and accelerated approval or fast track (allow antibiotics for serious conditions that filled an unmet medical need to be approved based on a surrogate endpoint)
4. Recommendations for hospitals - Where do things currently stand?
Four thematic areas to mitigate AMR in hospitals are as follows: i) antimicrobial stewardship practices, ii) appropriateness of therapy and adherence to treatment guidelines, protocols, and policies, iii) infection prevention control practices and iv) utilization of rapid diagnostic tests
Establishing antimicrobial stewardship team
Antimicrobial stewardship (ASP) is advocated to improve the quality of antimicrobial use. The Infectious Diseases Society of America/Society for Healthcare Epidemiology of America (IDSA/SHEA) guidelines3 identify two core proactive, evidence-based strategies for promoting antimicrobial stewardship [Figure 7]: i) formulary restriction and pre-authorization and ii) prospective audit with intervention and feedback.87 Supplemental Strategies include education, guidelines, and clinical pathways, antimicrobial order forms, streamlining or de-escalation, dose optimization, and intravenous to oral switch interventions considered as part of ASP are listed in Table 8. Several studies have demonstrated the positive impact of antimicrobial restriction policies and procedures; however, there is limited guidance on the optimal criteria and measures needed to develop an effective process and ensure adherence to the established policy.
- Infectious Diseases Society of America (IDSA) recommended antimicrobial stewardship strategies. IV to PO: Intravenous to oral..
| Intervention | Description/comment | Healthcare setting |
|---|---|---|
| Formulary restriction |
Antibiotics may be prescribed only:
|
inpatient/outpatient |
| Drug preauthorization | Permission (from ASP team member or infectious diseases specialist) required for release of certain antibiotics. often implemented together with formulary restriction | inpatient/outpatient |
| Prospective audit and feedback | Case review by trained asp team member and feedback of recommendations if reviewed antibiotics are deemed to be inappropriately prescribed. Labor-intensive. | inpatient |
| Prescriber education | More effective as a supplementary strategy to other interventions | inpatient/outpatient |
| Patient education | usually focus groups or mass media campaigns. | outpatient |
| Clinical guidelines | treatment protocols for various infections – may be institution-specific | inpatient/outpatient |
| Clinical decision support systems | Information technology systems for improving antibiotic prescription. Requires existing electronic records and electronic prescribing system to be effective | inpatient/outpatient |
| Point of care diagnostic tests | Diagnosis of non-bacterial etiologies may help reduce antibiotic prescription. | inpatient/outpatient |
| Microbiology laboratory susceptibility reporting | Selective reporting of susceptibility profiles for positive cultures may dramatically alter prescribing patterns of physicians | inpatient/outpatient |
| Antimicrobial cycling | Substitution of selected antibiotics over pre-defined periods. Little clear evidence for efficacy | inpatient |
Current stewardship efforts in India are few, and there is a significant dearth of trained personnel in most hospitals in addition to the absence of facility-specific antimicrobial guidelines and infection control measures.88 Pre-authorization for restricting high-end antibiotics has shown the beneficial effect of ASP in optimizing and reducing antibiotic use besides achieving improved patient outcomes.
Instituting a prior authorization program requires the ASP to consider many operational and logistic challenges. Because prompt antimicrobial therapy is extremely important in critically ill patients, approval logistics need to be addressed before starting a prior authorization program, particularly in facilities that cannot provide around-the-clock reviews.89 The Directorate General of Health Services (DGHS) recommends the establishment of a hospital pre-authorization committee for reviewing and approving watch and reserve category antibiotics to ensure that their usage aligns with established guidelines and justifies on the clinical needs.
Perioperative antibiotic prophylaxis is considered one of the most effective measures for the prevention of surgical site infections (SSIs). Restricting perioperative antibiotics increased the rational use of prophylactic antimicrobial use, with substantial improvement in the risk-benefit trade-off. A randomized control trial demonstrated that three doses of perioperative antibiotic are equally effective in preventing infective complications.90 Prospective audits and feedback (PAF) are often initiated based on the antimicrobials prescribed or by clinical results obtained from the microbiology laboratory. Post-prescription review (PPR) provides recommendations to change the agent, to adjust the dose or duration of therapy, to convert intravenous to oral formulations, and to evaluate drug–drug interactions.91 This review can be performed immediately after prescription or within 24–72 hours after prescription when more clinical information is available. In India, the implementation of PPR decreases antimicrobial use from 831.5 during the baseline phase to 717 DOT per 1000 in the intervention phase. Notably, 73.3% of antibiotic prescriptions were inappropriate, de-escalation according to culture susceptibility has improved significantly with PPR.88 Physicians and pharmacists trained in infectious diseases are ideally suited to perform PPR and to provide recommendations.
However, a lack of trained infectious disease physicians and pharmacists makes it challenging to establish antimicrobial stewardship in India, especially in public sector hospitals. DGHS also suggests conducting regular audits to evaluate the patterns and trends of antibiotic prescribing and usage at hospitals. This audit provides valuable insights into prescribing practices, identifies areas of improvement, and enables us to track progress in ASPs efforts.
A compilation of consumption in the form of defined daily doses (DDD) or days of therapy (DOT) may be used to determine high prescription areas to maximize the effect of interventions and this should be reviewed and updated over time.92 This strategy also provides opportunities for education through the feedback mechanism and promotes individualization of therapy. Compliance to institution-specific antibiotic guidelines showed marginal improvement in PPR intervention phase. Importantly, in 28.5% of the cases, antibiotic guidelines were not applicable, suggesting a need for comprehensive national- and institution-specific antibiotic policy guidelines based on the local antibiogram.92 The establishment of facility-specific treatment guidelines for optimizing empiric antimicrobial selection, de-escalation, duration of therapy, and also including recommendation of source control are warranted. In addition, continuous updates of the policy or treatment guidelines is necessary based on the national AMR surveillance data or cumulative antibiogram that reflects institution-specific antimicrobial usage.
A significant number of studies have assessed the quality of antibiotic prescribing in the outpatient settings. Up to 80–90% of antibiotics are prescribed in outpatient settings, with high levels of inappropriate use.93 Increasing attention has been paid to antibiotic stewardship efforts in the outpatient setting. Encouraging doctors to include clinical diagnosis and investigations in each case and the reason for antibiotic usage prescription by default helps identify the interventions that prevent inappropriate use. A major cause of misuse is insufficient knowledge of prescribing of antimicrobials in many categories of professionals, and education is the fundamental component of ASPs. Implementation of an education-based ASP was shown to improve antimicrobial prescriptions and consumption, even when restrictive measures were not implemented.94 However, current guidelines suggest that educational interventions should not be used alone but to support other stewardship interventions. A theme-based educational program provides a platform for stressing facility-specific issues and influencing prescribers’ behavior. Such interventions are most commonly directed toward prescribers and less likely educate pharmacists, nurses, or even members of the stewardship team.
Moreover, teaching of the principles of ASPs at the undergraduate and postgraduate level improves antibiotic use. Currently, in India, no structured provision of education and training exists for AMS.95
Studies evaluating the impact of implementing infection control and antibiotic stewardship practices on nosocomial infections are limited. In addition, the impact of studying the appropriateness of intervention measures in antimicrobial stewardship and infection control practices are urgently needed for Indian settings. Interestingly, a study from India has documented that improving basic infection control practices, rapid diagnostics, and antimicrobial stewardship practices as key tools for the reversal of AMR.96 There was a notifiable reduction in the incidence of priority pathogens, including VRE (43.5% in 2016 to 12.2% in 2021); carbapenem-resistant E. coli (21.6% in 2016 to 19.4% in 2021), carbapenem-resistant P. aeruginosa (23% in 2016 to 20.6% in 2021); carbapenem-resistant A. baumannii (66.6% in 2016 to 17% in 2021).The incidence of reduction in VRE and carbapenem-resistant A. baumannii was significant, but the reduction was found to be marginal in case of carbapenem-resistant E. coli and carbapenem-resistant P. aeruginosa. In addition, the rate of isolation of Candida spp. from non-sterile sites also showed a reduction, from 1.68 to 0.65 per 100 patients. Importantly, the incidence of HAIs also fell from 2.3 to 1.19 per 1000 line days for CLABSI and 2.28 to 1.88 per 1000 catheter days for complicated UTI.
In hospital or community, certain innovation strategies have been proposed to promote ASPs. It is an urgent appeal to all the doctors to make it a mandatory practice to write indication/reason/justification while prescribing antimicrobials. The OPD cards should be designed and structured to compel the clinicians to make note of clinical diagnoses and reasons for prescribing antibiotics. A person-centered practice for prescription can be promoted by recognizing the best prescriber team or individual. The essential recommendations are as follows:
-
Develop and maintain antimicrobial policies, procedures, and clinical pathways for antimicrobial treatment and prophylaxis.
-
Define and maintain a formulary restriction and approval process that include restricting broad-spectrum antimicrobials to patients in whom their use is clinically justified.
-
Develop and implement interventions and educational strategies for medical officers, nurses, pharmacists, and other clinical employees on appropriate antimicrobial prescribing and AMS principles, and monitor outcomes.
-
Maintain an awareness of local antimicrobial resistance patterns among local pathogens and relevant local outbreaks of infection, and to consider whether these may need to influence antimicrobial prescribing guidelines.
-
Monitor antimicrobial usage, including appropriateness of prescribing relative to evidence-based recommendations.
-
Participate in activities that permit benchmarking and comparisons of prescribing between facilities where appropriate and provide guidance on proper interpretation of these findings.
-
Ensure antimicrobial stewardship process, and outcome indicators are measured and reported to the hospital management and relevant committees.
-
Ensure that there is feedback of clinically relevant data regarding prescribing behaviors to prescribers and to other stakeholders, for example, nurses and pharmacists in a way that they can understand.
-
Ensure that APS activities are aligned with other hospital activities
-
Regular meetings should be established with defined objectives, action plans, and measurement of progress and outcomes. AMS committees will often meet monthly, or, for smaller centers, every second or third month. Any less often than quarterly (3 monthly) is rarely acceptable but might be relevant for a small center with a limited spectrum of diagnoses managed (e.g., a day-procedure hospital).
Recommendations for action
-
National Accreditation Board for Hospitals & Healthcare (NABH) accreditation should be made mandatory for licensure of all public and private hospitals: an AMSP program is one of the components of NABH accreditation.
-
An ID physician should be the convener for the AMSP program in all tertiary care hospitals: where no ID physician or department currently exists, a senior physician or surgeon or microbiologist can be the convener. In secondary and primary care hospitals, any physician or microbiologist with an interest in this area and who has a good rapport with other antimicrobial prescribers can be the convener.
Diagnostic stewardship
Inappropriate testing can also result in overdiagnosis of HAIs. Rapid and accurate diagnosis of infection is critical for appropriate antimicrobial initiation and subsequent optimization. The goal of diagnostic stewardship is to select the right test for the right patient, generating accurate, clinically relevant results at the right time to optimally influence clinical care and to conserve healthcare resources [Figure 8].97,98 The process of diagnostic stewardship begins with the evaluation, selection, and implementation of appropriate diagnostic tests for the clinical setting, incorporates guidance for health care providers regarding judicious use of testing for appropriate patients, and ensures timely sample collection, transport, and processing and timely reporting of results. Diagnostic stewardship and antimicrobial stewardship are both critical components of healthcare aimed at optimizing patient outcomes while minimizing the emergence of antimicrobial resistance. By integrating diagnostic stewardship with antimicrobial stewardship, healthcare providers can ensure that diagnostic tests are used judiciously to guide appropriate antimicrobial therapy, leading to improved patient outcomes and reduced AMR [Figure 9]. Key considerations and strategies for each step of the diagnostic stewardship process are outlined below and summarized in Table 9.
- Roles of diagnostic and antimicrobial stewardship in the implementation of rapid molecular infectious disease diagnostics in the clinical setting.
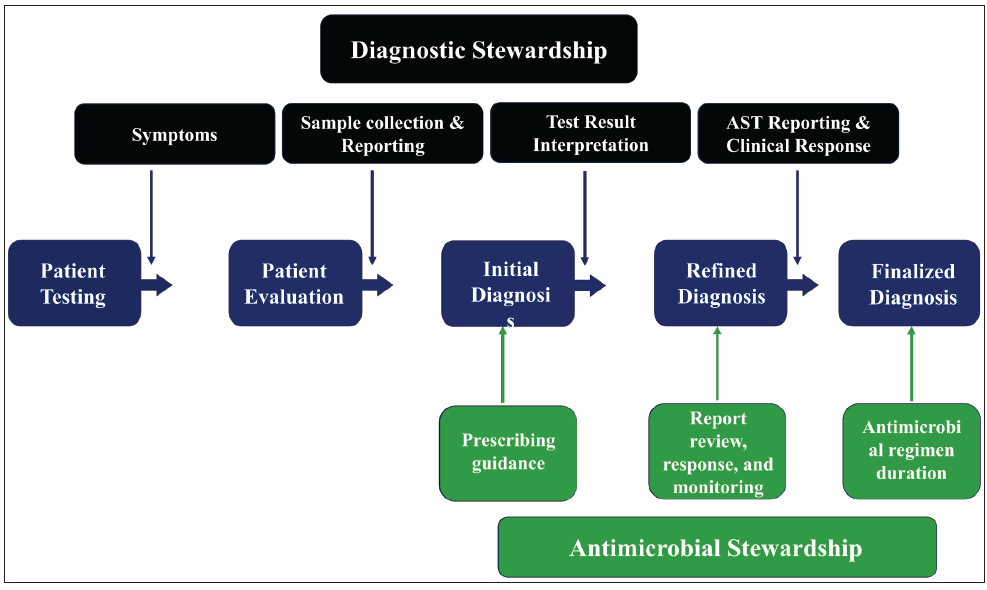
- Relationship between diagnostic stewardship (dark green) and antimicrobial stewardship (light green) on patient diagnosis and treatment.
| Goal | Key question | Key considerations and potential strategies |
|---|---|---|
| Right test | Is the test appropriate for the clinical setting? |
|
| Right patient | Will the clinical care of the patient be affected by the test result? |
|
| Right time | Will the result be available in time to optimally affect care? |
|
Diagnostic stewardship is more challenging for direct-from-specimen rapid diagnostic tests, for which the responsibility for appropriate ordering traditionally rests on the clinician. Development of diagnostic algorithms and inclusion of computerized order entry (CPOE) decision support can be used to direct clinicians toward the appropriate test for the clinical situation and curb unnecessary duplication of diagnostic testing.
Recommendation for action
-
All Microbiology laboratories (both stand-alone and hospital-associated) need to be mandatorily NABL accredited: this will automatically ensure a basic level of optimum practice and diagnostic stewardship
-
Diagnostic stewardship should be taught and emphasized in the core curriculum on Microbiology at the undergraduate and postgraduate levels.
Hospital infection control practices and NABH accreditation for tertiary care centers
Healthcare facilities are high-risk environments for the development and spread of resistant pathogens and frequently have the highest burden of multidrug-resistant pathogens, including carbapenem-resistant and difficult-to-treat gram-negative pathogens. In India, the burden of HAIs is high, with an estimated pooled prevalence of 15.5 per 100 patients.99 Most HAIs are preventable through effective infection prevention and control (IPC) measures [Figures 10 and 11]. They are, therefore important to efforts to contain AMR. At present, however, a lack of adequate systems and infrastructure for infection prevention and control in many healthcare facilities contributes to the development of HAIs and the spread of resistant pathogens.
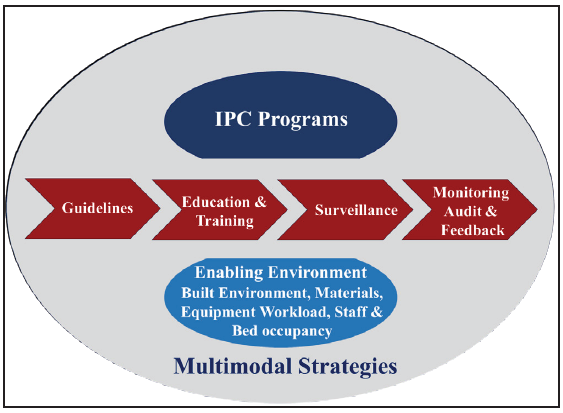
- Multimodal strategy for infection, prevention and control implementation and to improve IPC practices in hospitals. IPC: Infection prevention control.
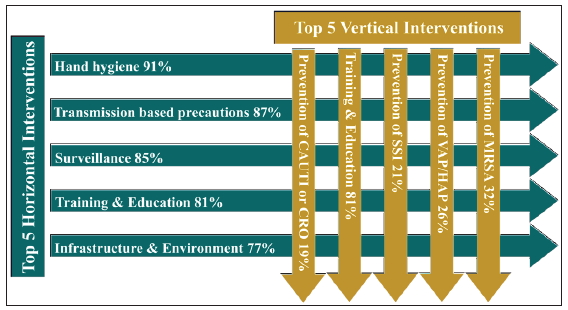
- Top 5 horizontal and vertical interventions suggested as a minimum requirement at the facility level.
Accreditation is an important strategy to improve and assure the quality of health care. Although hospital accreditation is not mandatory in India, groups such as the autonomous National Accreditation Board of Hospitals and the National Health Mission’s National Health Systems Resource Center have incorporated programs on infection prevention and control, including surveillance of HAIs, as a core part of the review and certification process.100,101 At the national level, there has been growing recognition of the need for policy and guidance documents, and in 2016 the ICMR released guidelines on infection prevention and control.102 Despite these initiatives, the successful implementation of an infection prevention and control program in Indian healthcare settings faces some important challenges, including insufficient funding and human resources, hospital overcrowding, and low nurse-to-patient ratios even in ICUs.103,104 Implementation and improvement of IPC programs are critical to reducing the impact of HAIs and the spread of multiresistant pathogens. The annual plan for prevention and control of HAIs are:
-
Actions to promote appropriate hygiene measures
-
Guidelines for empirical and targeted therapy based on national recommendations and local epidemiology, especially in life-threatening emergencies
-
Actions against the spread of nosocomial infections driven by specific local situations
-
Monitoring of alert microorganisms, including MRSA, VRE, ESBL-producing Enterobacterales, carbapenem-resistant isolates belonging to Enterobacterales, P. aeruginosa, and A. baumannii). The process starts from the microbiology laboratory: when an alert organism is isolated an alert is sent to the local IPC team.
-
Monitoring of antimicrobial use as provided by the hospital pharmacy with a focus on broad-spectrum antimicrobials
-
Annual one-point survey to establish the local prevalence of HAIs
-
Epidemiological reports on circulating pathogens and antimicrobial resistance
-
Educational annual program pointed to an appropriate antimicrobial use for all the hospital personnel
Active and passive surveillance programs should be implemented to assess and monitor the extent and trends of HAIs, inform alert and precautionary programs, and improve performance, strategy, and skill development. Active surveillance for multi-drug resistant organisms (MDROs) includes targeted screening to identify colonized patients on hospital admission which should be epidemiologically determined, such as performing entry rectal swabs for prevention and control of carbapenem-resistant Enterobacterales infections and nasal swabs for prevention and control of methicillin-resistant S. aureus in high-risk units.105 Active surveillance may be more directly associated with monitoring and controlling the risk of drug-resistant pathogen outbreaks. Monitoring should include all gram-negative and gram-positive organisms that represent a relevant threat according to local or national epidemiological assessment. Passive surveillance consists of data that are routinely generated from patient registration, laboratory or pharmacy data, or data from discharge. Quality microbiology and laboratory capacity are essential to enable reliable HAI surveillance, and laboratory reporting of alert organisms, usually multiresistant, is a due act of surveillance within the facility.106 The role of pharmacy and the antimicrobial stewardship team is to track trends in antibiotic consumption (DDD/100 hospital days) that is also measured for economic reporting purposes. Surveillance should provide information for:
-
Description of the status of HAIs (i.e., incidence and/or prevalence surveys, type, etiology, and ideally, data on severity and attributable burden of disease)
-
Identification of the most relevant AMR susceptibility patterns
-
Identification of high-risk populations, procedures, and exposures
-
Early detection of clusters and outbreaks (contact tracing)
-
Evaluation of the impact of interventions.
Education and training programs should be audited against predefined checklists that are revised over time to take into account local barriers and behavior.
Education and training should be combined with knowledge tests, competency assessments, or both. In order to reduce the incidence of nosocomial infections, compliance with interventions is mandatory.
Recommendations for action
-
Encouraging public hospitals to get NABH accreditation: a hospital infection control program is one of the NABH accreditation.
Promoting in-hospital formulary
An antimicrobial formulary is a simplified list of available antimicrobials, with accepted indications for use, dosing schedules, drug interactions, and adverse events. A robust formulary can allow for easier maintenance of guidelines, and provision of education and training. Available antimicrobials have been evaluated in a systematic manner and meet strict criteria for inclusion. This can benefit prescribers by limiting the number of antimicrobials that they will be learning how to utilize and therefore, should improve the appropriateness of prescribing [Figure 12]. The formulary should include a subset of restricted antimicrobials. The use of these restricted antimicrobials requires strict monitoring and adherence to the antimicrobial prescribing policy of the hospital. The WHO AWaRe classification of antibiotics could be used as the base for a formulary restriction policy, mainly targeting Watch and Reserve groups of antibiotics.107 Auditing compliance with the in-hospital formulary restriction is important to ensure the adherence of policies.
- Managing formularies in hospitals.
A hospital’s drug and therapeutic committee (DTC) has the responsibility of creating and managing the antimicrobial formulary list but may involve an antimicrobial subcommittee or rely on the AMS team to advise on the need for adding new antimicrobials or indications to the current list.108 The management of the antimicrobial formulary should involve periodical review at least monthly to quarterly would be preferable.109,110 This process should involve medical and pharmacy staff knowledgeable about the spectrum of activity of antimicrobials, pharmacokinetics, and pharmacodynamics, hospital antibiograms, and common infectious diseases. New antimicrobials should be added if they meet the criteria for inclusion, including acceptable data on safety, pharmacological action, adverse drug reactions, and drug interaction; reasons why this is superior to current formulary-listed antimicrobials; scientific evidence and literature to support its addition; updated clinical guidelines or treatment pathways; altered hospital infection patterns and antibiogram; acceptable cost-efficiency; and approved and quality source of supply.109 Current antimicrobials should be removed from the list if they meet the deletion criteria, including if the antimicrobial is no longer used; recent data on lack of safety and efficacy become available; the antimicrobial does not meet the requirements for cost-effectiveness if an acceptable alternative is identified.109 Antimicrobial formulary management includes:
-
The formulary should be consistent with any national formulary or approved standard infection treatment guidelines.
-
The formulary should be reviewed and revised periodically
-
Combination or fixed-dose antimicrobials should only be used in specific proven infections
-
The ability to prescribe antimicrobials is restricted to only those practitioners with appropriate prescribing skills
-
In Hospitals strict measures across the country to limit prolonging perioperative prophylaxis by stop orders after three IV doses and no oral doses. This should be audited.
Recommendations for action
-
NABH accreditation for licensure should be made mandatory for all public and private hospitals: optimum hospital formulary practice in regard to antibiotics is one of the components of NABH accreditation.
-
AMR and AMS should be introduced in the core curriculum of trainees in Pharmacy. Parenteral to oral switch of antibiotics should be rational and a nation-wide policy can be developed for this purpose
5. Interventions at clinical microbiology laboratory level
Integrating microbiology laboratories to ASPs is important in the area of diagnostic stewardship, the development of antibiograms to support optimal antibiotic use, the introduction of new diagnostic tests into the laboratory, the implementation of new antibiotic susceptibility testing interpretative criteria, and education of clinicians on laboratory testing practices. Strengthening clinical microbiology laboratories across the country in performing diagnosis and antimicrobial susceptibility testing, including rapid molecular diagnostics testing, supports ASPs [Figure 13]. Institution-wide antimicrobial resistance surveillance reported in the form of antibiograms informs decisions for empiric antimicrobial therapy, and timely and accurate patient-specific pathogen isolation, and susceptibility data inform directed antimicrobial therapy.
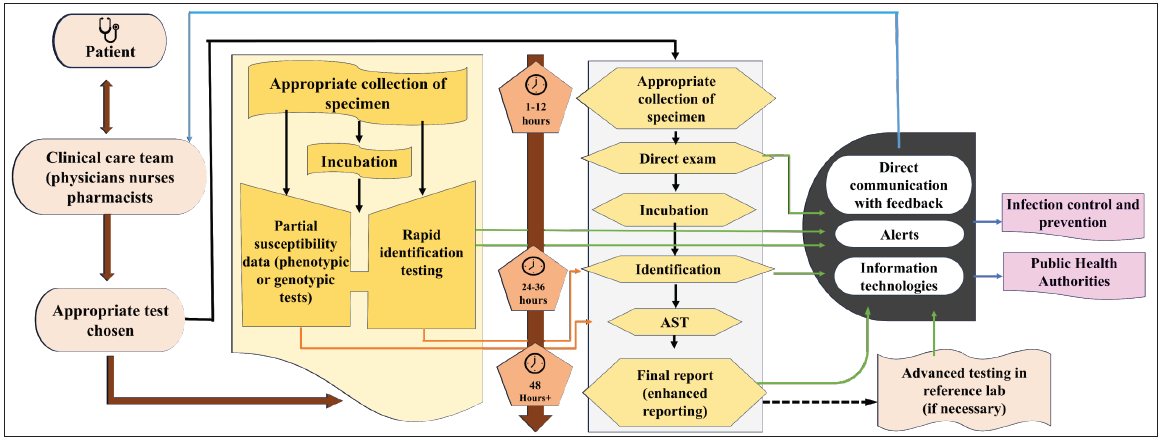
- Workflow pathways for conventional microbiology and RDT. Implementation of RDT increases laboratory workflow complexity but can hasten the availability of results. Communication of results is a key factor. Blue arrows represent the conventional microbiology pathway, orange arrows represent the RDT pathway, and green arrows represent opportunities for the laboratory and antimicrobial stewardship teams to improve communication of results. AST, antimicrobial susceptibility testing. AST: Antimicrobial Susceptibility testing, RDT: Rapid diagnostics tests.
Coupling clinical microbiology laboratory information with ASP interventions leads to the best antimicrobial use for individual patients and on an institutional level.
Maintaining high-quality clinical microbiology laboratories is the current best approach for diagnosis and treatment of infectious disease. The clinical microbiology laboratories, accredited under the National Accreditation Board for Testing and Calibration Laboratories (NABL), ensure quality infrastructure, laboratory operations, quality assurance, and continual improvement. Furthermore, regular participation in an external quality assessment scheme (EQAS) is crucial for ensuring acceptable laboratory performance in facilitating optimal patient care.
Tailoring susceptibility test performance and reporting to formulary decisions and the stewardship principle of encouraging narrower spectrum antibiotic use whenever possible is recommended. Use cascade reporting to promote preferential use of narrowest spectrum antibiotics [Figure 14].110 Update institution antibiograms following published guidance in the M39 CLSI document, at least on annual basis.111 Antibiogram helps the prescribers for selecting effective therapy when culture results are pending, and informing and update local guidelines for empirical treatment of common infection syndromes. From the antimicrobial stewardship standpoint, cascade or selective reporting can be used to promote the judicious use of antimicrobials. Cascades consist of algorithm-driven reports that provide only a limited number of tested antimicrobial susceptibilities based on formulary availability, local cumulative susceptibilities, and cost for isolates with no or low levels of resistance and reporting of susceptibility to broader-spectrum drugs only when isolates are resistant to drugs in the first “cascade.” Careful selection of reported susceptibilities and frequent re-evaluation are necessary to ensure the continued value and reliability of the cascade and the quality of the reporting. Unreleased susceptibility data should also be readily available upon clinician request.
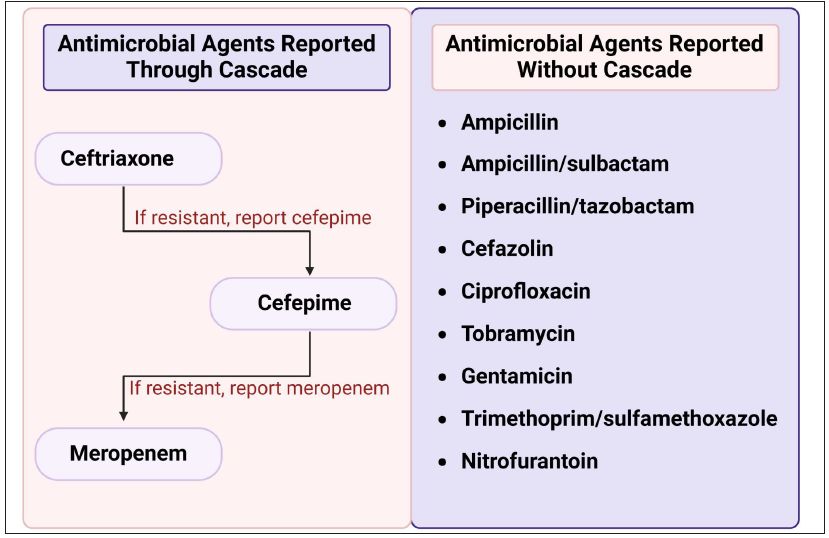
- Cascade reporting for Enterobacterales.
Molecular rapid diagnostic tests (mRDTs) have emerged as a key technology to decrease the time to identification and antimicrobial susceptibility of pathogens.91 These tests can decrease the time to effective therapy and antimicrobial optimization, and they also decrease the length of stay in patients with bloodstream infections. The clinical impact of mRDTs is most pronounced when reviewed and acted upon by the ASP in real time. Successful implementation of mRDTs is dependent on workflows supporting antimicrobial stewardship review of mRDTs results and on communicating recommendations to frontline clinicians.112,113
Incorporating mRDTs results into the ASP workflow should be a high priority in hospitals that utilize this technology.
Clinical microbiology laboratories are key to AMS programs, providing specimen collection and testing, rapid diagnostics, susceptibility testing, and production of antibiograms and education activities. The usefulness of clinical microbiology laboratories are as follows:
-
The clinical microbiology laboratory and the clinical microbiologist can play a pivotal role in any AMS program.
-
Clinical laboratories can provide diagnostic stewardship
-
The microbiology laboratory should ensure that all antimicrobial susceptibility results are reported in a selective manner; “cascade reporting” should be considered as part of an effective AMS program.
-
Early and direct communication with the treating team regarding the interpretation of results and offering clinical advice can help support AMS initiatives and optimize antimicrobial therapy.
-
Antibiograms can be used to help guide the local antimicrobial formulary, empiric antimicrobial guidelines and inform specific AMS interventions.
-
WHONET is a software program available from the WHO to assist in the development of cumulative antibiograms.
Recommendation for action
-
All Microbiology laboratories (both stand-alone and hospital-associated) need to mandatorily be NABL accredited: this will automatically ensure a basic level of quality control and optimal reporting.
-
State of the art microbiological practices that impact AMR should be included in the core curriculum of Microbiology at the undergraduate and postgraduate levels.
-
An early updated CLSI document should be distributed to all accredited laboratories by the government. This can prevent the use of obsolete breakpoints for susceptibility interpretation and therefore quality of AMR data can be maintained
6. Research and development
Promoting research for new antibiotics and rapid diagnosis
-
Promoting research for development of new antibiotics and validated point-of-care or rapid test for diagnosis of infectious disease can prevent misuse of antibiotics in the community.
New agents displaying innovative chemistry and modes of action are desperately needed worldwide to tackle AMR. Researchers developed an artificial intelligence (AI) model that designs novel, synthesizable antibiotic compounds, several of which showed potent in vitro activity against priority pathogens.114,115 Initially, researchers used predictive AI models to identify antimicrobial properties of existing drug compounds, but these models are not very efficient. Generative AI models can go a step further to design brand new drugs, but these tend to be difficult to synthesize. However, additional studies on the newly identified antibiotics are warranted. While there is room for growth and improvement, India’s position in antibiotic development is poised for advancement. With its strong pharmaceutical industry, research capabilities, and ongoing initiatives, India has the potential to play a significant role in addressing the global challenge of AMR through the development of novel effective antibiotics.
Lack of easy access to diagnostic testing, in fact, has made the diagnostic step one of the weakest links in the cascade of patient care. Moreover, timely and accurate diagnosis through rapid tests can reduce the time to pathogen identification and facilitate faster, optimized antimicrobial treatment. The most common identified gaps include inadequate near-patient testing for identification, susceptibility testing and biomarkers.116 The R&D priority for rapid diagnostics against AMR includes
-
Improved near-patient testing for ID and susceptibility
-
Host response tests, identifying biomarkers
-
Multiplex diagnostic platform to identify bacterial pathogens and perform susceptibility testing directly from samples
-
Simple, easy-to-use test or platform for antimicrobial susceptibility testing (AST) only
Recommendations for action
-
Both investigators initiated and ICMR-initiated projects concerning diagnostics and therapeutics impacting AMR need to be accorded priority with fast-track approval and increased funding.
Promoting one-health approach
One Health is an integrated approach that recognizes the health of humans, animals, and the wider environment as closely linked and interdependent [Figure 15].117 It requires multidisciplinary collaboration, adequate surveillance systems, and strong laboratory capacity, many of which are challenges for Indian settings. Several integrated approaches have been proposed to reduce antibiotic misuse in human, environmental, and animal health [Table 10].118
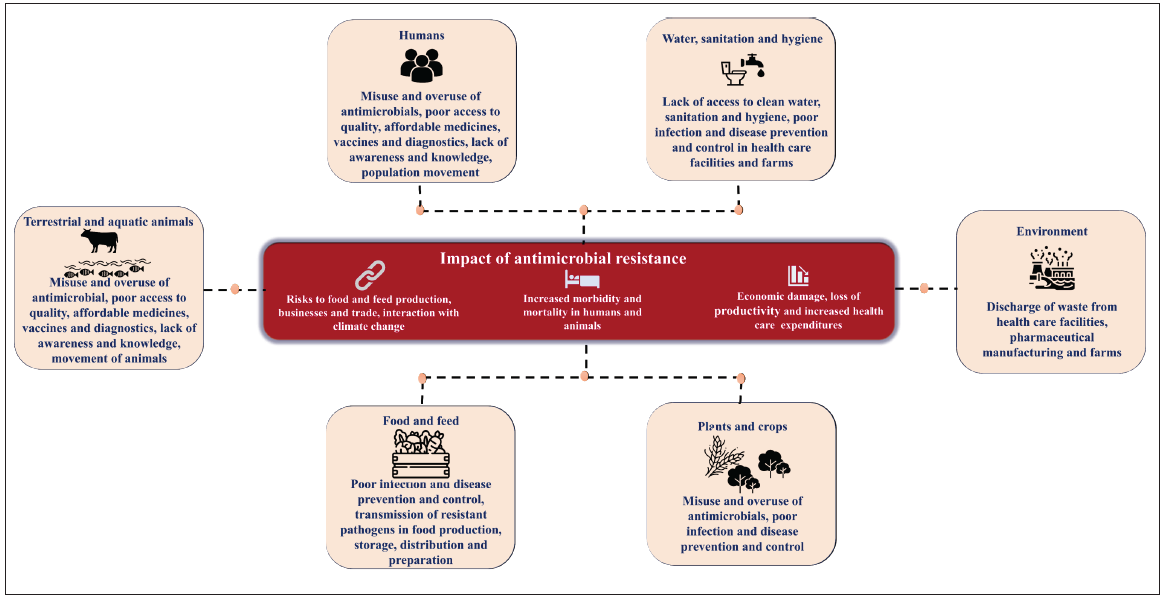
- Drivers of antimicrobial resistance (AMR) in one health aspect.
| Strategies | Specific actions |
|---|---|
| Manage antibiotic use in animal agriculture | Survey antibiotic use in livestock and aquaculture production |
| Create antibiotic resistance action plans that address antibiotic use in agriculture | |
| Improve farmer education about best practices in animal agriculture, including vaccine use and antibiotic withdrawal periods before slaughter | |
| Improve waste disposal from farms | |
| Ban use of antibiotics that are critically important to human health eg cephalosporins, fluoroquinolones, tetracyclines, colistin | |
| Livestock antimicrobial resistance (AMR) surveillance | Generating surveillance-based AMR data from the veterinary practice is required to counteract resistance and preserve the efficacy of antimicrobial agents |
| Treat highly contaminated waste effluent before disposal | Enforce minimum treatment levels for hospital and drug manufacturer waste |
| Improve access to clean water and sanitation | Consider water and sanitation initiatives |
Restricting the use of antibiotics in food-producing animals
There is growing concern that nonprudent use of antibiotics is linked with the escalating emergence of human infections with antibiotic-resistant pathogens of zoonotic importance. In farm animals, antibiotics are commonly used to promote more rapid growth quickly, however, there is evidence to suggest that use of antibiotics at low- or sub-therapeutic levels fosters the development of resistant bacteria. Additionally, high levels of antibiotic-resistant genes have also been identified in soils fertilized with manure and river waters contaminated by runoff from animal farms. Therefore, the presence of resistance and residues of antimicrobials in foods of animal origin constitutes a potential risk to the health of consumers and are also recognized as an emerging environmental problem. In India, regulations controlling the use of antibiotics in animals are very weak and in the initial phases.
Low doses of antibiotics to promote growth are often used to compensate for poor farm hygiene and crowded conditions.119 In India, projected financial losses for a proposed ban on subtherapeutic antibiotic use were 1–3% of annual meat production, with the greatest losses by poultry farmers.120
Fewer intervention strategies have been proposed to address the contributions of animal reservoirs to the dissemination of antibiotic resistance. First, antibiotic use in the livestock and aquaculture industries should be evaluated.121 Little is known about the quantity, frequency of administration, or types of antibiotics used in animal production. Survey results can be used to identify which types or aspects of animal production are most in need of oversight, and once identified, comprehensive monitoring programs could be designed to address them. A systematic review and meta-analysis showed that reducing antibiotic use decreased the prevalence of antibiotic-resistant bacteria in animals by about 15% and multidrug-resistant bacteria by 24–32%.122 Second, administration practices that result in veterinary drug residues at slaughter must be restricted.123 Third, any antibiotic classes that are of critical importance to human health (e.g., colistin) should be banned for animal use.119 Fourth, considering alternative options to using antibiotics for disease prevention in animals include improving hygiene, better use of vaccination, and changes in animal housing and husbandry practices.124 Importantly, farmer education is also necessary to reduce animal antibiotic misuse.
Recommendations for action
Interventions strategies that help to reduce the need of antimicrobials in farm animals are as follows:
-
Restricted preventive use of antimicrobials both in groups of animals and medicated feed
-
Restriction on metaphylactic use of antimicrobials
-
Antimicrobials should be strictly avoided for the purpose of promoting growth and increasing yield
-
Reserve certain antibiotics for humans only. A list of such antibiotics may be drawn up after inputs from medical and veterinary experts.
-
Make obligatory for state government to collect data on antibiotic sale and use of antimicrobials in farm animals
Interventions to reduce environmental antimicrobial resistance
The environment acts as a mixing pot of drug-resistant bacteria from many sources such as pharmaceutical, biomedical, veterinary, and agricultural sectors. There are three main emission sources in the environment includes animal farming, hospital or community sewage, and pharmaceutical industry. Wastewater discharge from antimicrobial production is a hotspot for AMR development. Because antibiotics present in pharmaceutical wastewater have not been metabolized, their concentrations may be manyfold higher than in human waste.125 Subsequently, a study on wastewater treatment plant treating discharge from 90 pharmaceutical companies in India has reported to release a huge amount of ciprofloxacin in 1 day that equals to an amount prescribed to humans in Sweden over 5 days.126
Additionally, hospitals discharge antibiotic-resistant bacteria, which propagate in the hospital setting due to poor infection control.119 Moreover, inadequate sanitation and poor hygiene practices amplify the propensity for increasing antimicrobial pollution in the environment. Thus, prompt interventions are urgently needed to address the emergence and transmission of AMR in the environment.
Wastewater serves as a pivotal juncture for the dissemination of antibiotic-resistant organisms and functions as a pathway through which strains of human and animal origin can infiltrate the environment and potentially colonize new hosts. Even if present, the wastewater treatment plants are not designed to remove antibiotics or antibiotic resistance genes. The proposed predicted no effect concentrations (PNECs) for resistance selection ranges from 8 ng/L to 64 μg/L.127 However, the lack of optimal wastewater treatment increases its overall risk in India. The high cost associated with regular monitoring of antimicrobial levels in pharmaceutical waste water makes it a low-priority objective, and a strict vigilance on the effluent produced is also needed.128 Pharmaceutical companies may be able to reduce antibiotic discharge by improving manufacturing practices and on-site industrial waste treatment systems.129 Noncompliance penalties, such as fines or revocation of operation permits, may be necessary for enforcement.
Hospital wastewater containing disinfectants, antibiotics, and microbial and organic compounds can be very harmful for the environment and humans. The existing evidence indicates inadequate microbial treatment emphasizing the need for improvement in healthcare waste management. Over the years, various treatment technologies, including biological methods, such as activated sludge process, membrane bioreactor, moving bed bioreactor, constructed wetlands, the advanced oxidation processes, such as photocatalysis and Fenton process, have been implemented to treat the effluents. Noticeably, high proportions of multidrug-resistant and carbapenem-resistant gram-negative pathogens have been reported in the treated hospital effluents in India.130–133
Recommendations for action
The following are the interventions to prevent the dissemination of resistant pathogens and release of antibiotic residues into the environment.
-
Decontamination of human and animal wastewater and sewage from hospitals, farms, urban sewage, treatment plant discharge, sewage overflow, run-off manure-fertilized agricultural fields and livestock farms
-
Surface microbial decontamination of floors and equipment in hospitals and farms.
-
Prevention of environmental releases and decontamination of antimicrobial substances, including biocides, metals, and industrial pollutants, as influencing the environmental selection of resistant organisms
-
Sublethal concentrations of biocides can increase the pool of resistant organisms in the environment. Another important aspect is the sharing of resistance mechanisms between biocides and antimicrobial agents, thus facilitating their co-selection. Biocides as a route of AMR need to be listed in the NAP-AMR of India.
Inequity in priorities and agendas between the Global North and the Global South
The term “Global North” typically refers to the more economically developed countries, while the “Global South” encompasses less economically developed countries. The division between these regions extends beyond economic factors and influences priorities and agendas related to AMR. In the Global North, where access to healthcare and resources for research and development is generally higher, there is often a greater focus on developing new antimicrobial drugs and technologies to combat resistance. However, this approach may not be feasible or effective in the Global South due to financial constraints and different healthcare infrastructures.
Conversely, the Global South often faces more immediate challenges related to access to basic healthcare, sanitation, and clean water, which are critical for preventing infections in the first place. Therefore, their priorities may lean toward strengthening healthcare systems, improving sanitation, and promoting responsible antimicrobial use to prevent the spread of resistance. This disparity in priorities and agendas between the Global North and South poses a significant threat to global efforts to mitigate AMR. Collaboration and coordination between these regions are essential to address the multifaceted challenges posed by AMR effectively.
Thera are three examples that illustrate these differences. In European countries, a direct correlation between outpatient penicillin use and the S. pneumoniae resistance rate to penicillin has been demonstrated. However, in a study on low-income countries, the direct association between resistance to and use of fluoroquinolones or third-generation cephalosporins on E. coli was not demonstrated. However, a study on LMICs demonstrated no direct association between resistance to and use of fluoroquinolones or third-generation cephalosporins on E. coli. This study examined the impact of universally applicable interventions such as governance, health expenditure, gross domestic product per capita, education, infrastructure, and climate on AMR. The findings indicated that infrastructure interventions, which included adequate sanitation, access to improved water sources and electricity, and urbanization, contributed significantly to the reduction in AMR. Further, environmental sources, such as water, soil, and surfaces within the household, are critical reservoirs for ESBL-producing bacteria. These contaminated environments facilitate the transmission of resistant bacteria between animals and humans. This research underscores the interconnectedness of human, animal, and environmental health, a concept central to the One Health approach. Further, no correlation was observed between country-level antibiotic usage and total AMR gene abundance, suggesting that non-antibiotic-use factors play a more significant role in driving the presence of AMR genes in sewage.
The danger of the Global South falling short of opportunities to fundamentally mitigate AMR is further exacerbated by the focus of global funding toward research and development of new antibiotics. However, there is a risk of neglecting these holistic and preventive measures, which are vital for sustainable AMR mitigation. A balanced approach that includes significant investments in these areas is essential to effectively combat AMR, especially in the Global South. Less funding for vaccines for research and development in diagnostics further complicate AMR issue in the Global South. The inequality in global pharmaceutical procurement, where the Global North dominates over 60% of the market, contrasts sharply with antibiotic production, which is primarily concentrated in countries like China and India. This distribution poses significant environmental risks, particularly concerning water contamination, due to the discharge of antibiotic residues into nearby water sources. Such disparities underscore the complexities of the global pharmaceutical landscape, highlighting not only economic divides but also environmental concerns that require attention and cooperation on a global scale.
Recommendation for action
-
The United Nations Secretary General play a pivotal role in accelerating the establishment and funding of this panel by advocating for its importance on the global stage, Additionally, leveraging existing platforms and initiatives within the UN system, such as the WHO and the Food and Agriculture Organization (FAO), could amplify efforts to address AMR holistically.
-
By prioritizing AMR, G77 leaders can take proactive measures to mitigate these risks and promote sustainable development. This includes strengthening healthcare systems, promoting responsible antibiotic use in agriculture, investing in research and development of new antimicrobial drugs, and fostering international collaboration to address this urgent threat.
-
Linking equitable AMR mitigation interventions to the attainment of country-level sustainable development goals (SDGs)
-
Negotiations on knowledge transfer and capacity development for antimicrobials, vaccines, and diagnostics should indeed involve the UN, given its convening power and its ability to coordinate efforts among member states.
References
- Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629-655.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Annual report of Antimicrobial resistance Research and Surveillance Network, January to December 2022. ICMR, Division of Epidemiology and Communicable Disease; 2023. https://main.icmr.nic.in/sites/default/files/upload_documents/AMRSN_Annual_ Report_2022.pdf. [Last accessed 2024 Sept 3]
- National Antimicrobial Surveillance Network (NARS-Net), National Programme on AMR Containment, January to December 2022. 2023, https://www.ncdc.gov.in/WriteReadData/l892s/1257263841692628161.pdf. [Last accessed 2024 Sept 3].
- First Indian report on genome-wide comparison of multidrug-resistant escherichia coli from blood stream infections. PLoS One. 2020;15:e0220428.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Distinctive mobile genetic elements observed in the clonal expansion of carbapenem-resistant klebsiella pneumoniae in India. Microb Drug Resist. 2021;27:1096-1104.
- [CrossRef] [PubMed] [Google Scholar]
- Performance standards for antimicrobial testing (32nd ed, CLSI supplement M100). Wayne, PA: Clinical and Laboratory Standards Institute; 2022.
- Expected plazomicin susceptibility in India based on the prevailing aminoglycoside resistance mechanisms in Gram-negative organisms derived from whole-genome sequencing. Indian J Med Microbiol. 2020;38:313-318.
- [CrossRef] [PubMed] [Google Scholar]
- The use of cefepime for treating ampC β-lactamase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57:781-8.
- [CrossRef] [PubMed] [Google Scholar]
- Infectious diseases society of america guidance on the treatment of ampc β-lactamase-producing enterobacterales, carbapenem-resistant acinetobacter baumannii, and stenotrophomonas maltophilia infections. Clin Infect Dis. 2022;74:2089-2114.
- [CrossRef] [PubMed] [Google Scholar]
- Dominance of international high-risk clones in carbapenemase-producing Pseudomonas aeruginosa: Multicentric molecular epidemiology report from India. Indian J Med Microbiol. 2018;36:344-351.
- [CrossRef] [PubMed] [Google Scholar]
- Performance standards for antimicrobial testing (33rd ed, CLSI supplement M100). Wayne, PA: Clinical and Laboratory Standards Institute; 2023.
- Polymyxins resistance among Gram-negative pathogens in India. Lancet Infect Dis. 2020;20:1362-1363.
- [CrossRef] [PubMed] [Google Scholar]
- Enhanced bacterial killing with a combination of sulbactam/minocycline against dual carbapenemase-producing acinetobacter baumannii. Eur J Clin Microbiol Infect Dis. 2023;42:645-651.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acinetobacter baumannii: An Emerging and important pathogen. J Clin Outcomes Manag. 2010;17:363-369.
- [PubMed] [PubMed Central] [Google Scholar]
- Is it time to move away from polymyxins?: Evidence and alternatives. Eur J Clin Microbiol Infect Dis. 2021;40:461-475.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- In vitro activity of two cefepime-based novel combinations, cefepime/taniborbactam and cefepime/zidebactam, against carbapenemase-expressing enterobacterales collected in India. Microbiol Spectr. 2023;11:e0492522.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Susceptibility testing for aztreonam plus ceftazidime/avibactam combination: A general guidance for clinical microbiology laboratories in India. Indian J Med Microbiol. 2022;40:3-6.
- [CrossRef] [PubMed] [Google Scholar]
- Management of serious infections caused by metallo β-lactamases with or without OXA-48-like expressing Enterobacterales with aztreonam and ceftazidime/avibactam combination: Dosing strategy for better clinical outcome. Indian J Med Microbiol. 2021;39:286-288.
- [CrossRef] [PubMed] [Google Scholar]
- High prevalence of Escherichia coli clinical isolates in India harbouring four amino acid inserts in PBP3 adversely impacting activity of aztreonam/avibactam. J Antimicrob Chemother. 2020;75:1650-1651.
- [CrossRef] [PubMed] [Google Scholar]
- Occurrence of high levels of cefiderocol resistance in carbapenem-resistant escherichia coli before its approval in china: A report from China CRE-network. Microbiol Spectr. 2022;10:e0267021.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Genetic features leading to reduced susceptibility to aztreonam-avibactam among metallo-β-lactamase-producing escherichia coli isolates. Antimicrob Agents Chemother. 2020;64:e01659-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence and genetic mechanisms of antimicrobial resistance in Staphylococcus species: A multicentre report of the indian council of medical research antimicrobial resistance surveillance network. Indian J Med Microbiol. 2017;35:53-60.
- [CrossRef] [PubMed] [Google Scholar]
- Genomic insights on heterogeneous resistance to vancomycin and teicoplanin in methicillin-resistant staphylococcus aureus: A first report from South India. PLoS One. 2019;14:e0227009.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Detection of heterogeneous vancomycin-intermediate Staphylococcus aureus: A preliminary report from south India. Indian J Med Res. 2019;150:194-198.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Novel linear plasmids carrying vanA cluster drives the spread of vancomycin resistance in Enterococcus faecium in India. J Glob Antimicrob Resist. 2022;29:168-172.
- [CrossRef] [PubMed] [Google Scholar]
- Genomic insights of optrA-carrying linezolid-resistant Enterococcus faecium using hybrid assembly: First report from India. J Glob Antimicrob Resist. 2021;25:331-336.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med. 2015;41:285-95.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and clinical outcomes of invasive mould infections in Indian intensive care units (FISF study) J Crit Care. 2019;51:64-70.
- [CrossRef] [PubMed] [Google Scholar]
- Candida auris candidaemia in Indian ICUs: Analysis of risk factors. J Antimicrob Chemother. 2017;72:1794-1801.
- [CrossRef] [PubMed] [Google Scholar]
- Candida auris candidaemia in an intensive care unit - Prospective observational study to evaluate epidemiology, risk factors, and outcome. J Crit Care. 2020;57:42-48.
- [CrossRef] [PubMed] [Google Scholar]
- Candidaemia in a tertiary care centre of developing country: Monitoring possible change in spectrum of agents and antifungal susceptibility. Indian J Med Microbiol. 2020;38:110-116.
- [CrossRef] [PubMed] [Google Scholar]
- Emergence of clonal fluconazole-resistant Candida parapsilosis clinical isolates in a multicentre laboratory-based surveillance study in India. J Antimicrob Chemother. 2019;74:1260-1268.
- [CrossRef] [PubMed] [Google Scholar]
- Expert Consensus on The Management of Dermatophytosis in India (ECTODERM India) BMC Dermatol. 2018;18:6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The great indian epidemic of superficial dermatophytosis: An appraisal. Indian J Dermatol. 2017;62:227-236.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A prospective study of the epidemiological and clinical patterns of recurrent dermatophytosis at a tertiary care hospital in India. Indian J Dermatol Venereol Leprol. 2018;84:678-684.
- [CrossRef] [PubMed] [Google Scholar]
- A study of in vitro antifungal susceptibility patterns of dermatophytic fungi at a tertiary care center in Western India. Indian J Dermatol. 2019;64:277-284.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mutation in the squalene epoxidase gene of trichophyton interdigitale and trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother. 2018;62:e02522-17.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses. 2018;61:477-484.
- [CrossRef] [PubMed] [Google Scholar]
- Alarming India-wide phenomenon of antifungal resistance in dermatophytes: A multicentre study. Mycoses. 2020;63:717-728.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-myocological profile of tinea capitis in North India and response to griseofulvin. J Dermatol. 2001;28:22-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob Agents Chemother. 2003;47:82-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology and clinical outcomes of invasive mould infections in Indian intensive care units (FISF study) J Crit Care. 2019;51:64-70.
- [CrossRef] [PubMed] [Google Scholar]
- Azole-resistant aspergillosis: Epidemiology, molecular mechanisms, and treatment. J Infect Dis. 2017;216:S436-S444.
- [CrossRef] [PubMed] [Google Scholar]
- High-frequency direct detection of triazole resistance in aspergillus fumigatus from patients with chronic pulmonary fungal diseases in India. J Fungi (Basel). 2020;6:67.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tackling AMR from a multidisciplinary perspective: A primer from education and psychology. Int Microbiol. 2023;26:1-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115:E3463-E3470.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Strengthening the science of addressing antimicrobial resistance: A framework for planning, conducting and disseminating antimicrobial resistance intervention research. Health Res Policy Syst. 2020;18:60.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Developing and evaluating complex interventions: The new medical research council guidance. BMJ. 2008;337:a1655.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Exploring the evidence base for national and regional policy interventions to combat resistance. Lancet. 2016;387:285-95.
- [CrossRef] [PubMed] [Google Scholar]
- India’s national action plan on antimicrobial resistance: A critical perspective. J Glob Antimicrob Resist. 2021;27:236-238.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic Awareness Week Q&A—Dr Sumanth Gandra. https://blogs. biomedcentral.com/on-health/2020/11/17/antibiotic-awareness-week-qa- drsumanth-gandra/ [Last accessed 2021 Oct 22].
- Establishing antimicrobial resistance surveillance & research network in India: Journey so far. Indian J Med Res. 2019;149:164-179.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Country data on AMR in India in the context of community-acquired respiratory tract infections: Links between antibiotic susceptibility, local and international antibiotic prescribing guidelines, access to medicine and clinical outcome. J Antimicrob Chemother. 2022;77:i10-i17.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Global and regional burden of attributable and associated bacterial antimicrobial resistance avertable by vaccination: Modelling study. BMJ Glob Health. 2023;8:e011341.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Impact of pneumococcal conjugate vaccine and of reduction of antibiotic use on nasopharyngeal carriage of nonsusceptible pneumococci in children with acute otitis media. Pediatr Infect Dis J. 2006;25:1001-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention of antimicrobial prescribing among infants following maternal vaccination against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2022;119:e2112410119.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Reducing antibiotic use in influenza: Challenges and rewards. Clin Microbiol Infect. 2008;14:298-306.
- [CrossRef] [PubMed] [Google Scholar]
- Vaccines Europe. Vaccines Europe reveals its oirst pipeline review. [Online].; 2022. Available from: https://www.vaccineseurope.eu/news/articles/vaccines-europe-reveals-its- oirst-pipeline-review. [Last accessed 2023 Jan 6]
- The role of vaccines in combatting antimicrobial resistance. Nat Rev Microbiol. 2021;19:287-302.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Public health interventions to improve antimicrobial resistance awareness and behavioural change associated with antimicrobial use: A systematic review exploring the use of social media. Antibiotics (Basel). 2022;11:669.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Community engagement: The key to tackling Antimicrobial Resistance (AMR) across a One Health context? Glob Public Health. 2022;17:2647-2664.
- [CrossRef] [PubMed] [Google Scholar]
- India lauded for red line campaign on antibiotics. The Hindu 2016 Available from:https://www.thehindu.com/news/x/india-lauded-for-red-line-campaign- on-antibiotics/article8622474.ece. [Last accessed on 2021 Apr 10]
- [Google Scholar]
- The role of Schedule H1 and Red Line campaign in improving antibiotic use in India. J Family Med Prim Care. 2022;11:2656-2661.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Educational interventions to improve antibiotic use in the community: Report from the International Forum on Antibiotic Resistance (IFAR) colloquium, 2002. Lancet Infect Dis. 2004;4:44-53.
- [CrossRef] [PubMed] [Google Scholar]
- Changing public attitudes to antibiotic prescribing: Can the internet help? Inform Prim Care. 2004;12:19-26.
- [CrossRef] [PubMed] [Google Scholar]
- Co-designing community-based interventions to tackle antimicrobial resistance (AMR): What to include and why. BMC Res Notes. 2023;16:290.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- India’s national action plan on antimicrobial resistance: A critical perspective. J Glob Antimicrob Resist. 2021;27:236-238.
- [CrossRef] [PubMed] [Google Scholar]
- Global action plan on antimicrobial resistance. Geneva: World Health Organization; 2015. Available from: https://iris.who.int/bitstream/handle/10665/252683/A69_24-en.pdf?sequence=1 [Last accessed 2024 Feb 05]
- WHO competency framework for health workers’ education and training on antimicrobial resistance. World Health Organization; 2018. https://iris.who.int/handle/10665/272766
- Introduction of antimicrobial resistance education in pharmacy curricula in India: lessons learned and future direction. Clinical Epidemiology and Global Health 2024 Apr 16:101614.
- [CrossRef] [Google Scholar]
- Health workers’ education and training on antimicrobial resistance: curricula guide. World Health Organization; 2019. https://iris.who.int/handle/10665/329380. [Last accessed 2024 Sept 3]
- Systemic antibiotic sales and WHO recommendations, India. Bull World Health Organ. 2022;100:610-619.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- WHO AWaRe Classioication Database of Antibiotics for evaluation and monitoring of use [internet]. Geneva: World Health Organization; 2019. Available from: https://www.who.int/publications/i/item/WHOEMPIAU2019 .11 [Last accessed 2024 Sept 3]
- Use of fixed dose combination (FDC) drugs in India: central regulatory approval and sales of FDCS containing Non-Steroidal Anti-Inflammatory Drugs (NSAIDS), metformin, or psychotropic drugs. PLoS Med. 2015;12:e1001826. discussion e1001826
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Access, Watch, and Reserve antibiotics in India: Challenges for WHO stewardship. Lancet Glob Health. 2017;5:e1075-e1076.
- [CrossRef] [PubMed] [Google Scholar]
- The Gazette of India. New Delhi: Ministry of Health and Family Welfare; 2016. Available from: https://cdsco.gov.in/opencms/opencms/system/modules/ CDSCO.WEB/elements/download_oile_division.jsp?num_id=MTA2MQ== [Last accessed 2022 May 31]
- Report of Expert Committee on Fixed Dose Combinations (FDCs) Licensed by SLAs for manufacture without approval of DCG(I), applications of which have been received by CDSCO. New Delhi: Kokate Committee; 2015. Available from: https://cdsco.gov.in/opencms/opencms/system/modules/ [Last accessed 2024 Sept 3]
- CDSCO.WEB/elements/common_download.jsp?num_id_pk=NjU. Report of Expert Committee on Applications of Fixed dose Combinations (FDCs) received by CDSCO for proving safety and efoicacy categorized under category ‘a’. New Delhi: Kokate Committee; 2015. Available from: https://www.cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/Upload CommitteeFiles/fdc16.04.2015.pdf . [Last accessed 2022 Jul 14]
- FDCs requiring further deliberation with subject experts. New Delhi: Central Drugs Standard Control Organization, Ministry of Health & Family Welfare, Kokate Committee; 2016. Available from: https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadCom mitteeFiles/1fdc27-5-2016.pdf [Last accessed 2024 Sept 3]
- Over-the-counter sales of antibiotics for human use in India: the challenges and opportunities for regulation. Antibiotics (Basel). 2021;10(9):1123. PMID: 34572705
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The role of Schedule H1 and Red Line campaign in improving antibiotic use in India. J Family Med Prim Care. 2022;11:2656-2661.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Over-the-counter sale of antibiotics in India: A qualitative study of providers’ perspectives across two states. Antibiotics (Basel). 2021;10:1123.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Drugs and Cosmetics Rules 1945, Rules for Retailers. Available online: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf- documents/acts_rules/2016DrugsandCosmeticsAct1940Rules1945.pdf [Last accessed 2021 Sept 8].
- An evaluation of drug lag for new drugs approved by the Indian regulator relative to the United States, European Union, and Japanese regulatory agencies: A 15-year analysis (2004-2018) Perspect Clin Res. 2021;12:159-164.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- New drugs and clinical trials rules, 2019: Towards fast-track accessibility of new drugs to the Indian population. Ind J Pharm Educ. 2019;53:s451-9.
- [CrossRef] [Google Scholar]
- Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159-77.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of an antimicrobial stewardship intervention in India: Evaluation of post-prescription review and feedback as a method of promoting optimal antimicrobial use in the intensive care units of a tertiary-care hospital. Infect Control Hosp Epidemiol. 2019;40:512-519.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of a hospital-based antimicrobial management program on clinical and economic outcomes. Clin Infect Dis. 2001;33:289-95.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized control trial on perioperative antibiotic prophylaxis in live liver donors: Are three doses enough? J Hepatobiliary Pancreat Sci. 2022;29:1124-1132.
- [CrossRef] [PubMed] [Google Scholar]
- Interventions to optimize antimicrobial stewardship. Antimicrob Steward Healthc Epidemiol. 2021;1:e46.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antimicrobial stewardship: A review of prospective audit and feedback systems and an objective evaluation of outcomes. Virulence. 2013;4:151-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Advances in optimizing the prescription of antibiotics in outpatient settings. BMJ. 2018;363:k3047.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Global impact of an educational antimicrobial stewardship programme on prescribing practice in a tertiary hospital centre. Clin Microbiol Infect. 2014;20:82-8.
- [CrossRef] [PubMed] [Google Scholar]
- The state of education and training for antimicrobial stewardship programs in Indian hospitals-A qualitative and quantitative assessment. Antibiotics (Basel). 2019;8:11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Impact of improving infection control and antibiotic stewardship practices on nosocomial infections and antimicrobial resistance in an oncology centre from India. Indian J Med Microbiol. 2023;45:100383.
- [CrossRef] [PubMed] [Google Scholar]
- Leveraging diagnostic stewardship within antimicrobial stewardship programmes. Drugs Context. 2023;12:2022-9-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Implementation of rapid molecular infectious disease diagnostics: The role of diagnostic and antimicrobial stewardship. J Clin Microbiol. 2017;55:715-723.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Healthcare-associated infection in developing countries: Simple solutions to meet complex challenges. Infect Control Hosp Epidemiol. 2007;28:1323-7.
- [CrossRef] [PubMed] [Google Scholar]
- Healthcare-associated infection in developing countries: Simple solutions to meet complex challenges. Infect Control Hosp Epidemiol. 2007;28:1323-7.
- [CrossRef] [PubMed] [Google Scholar]
- National Quality Assurance Standards Accredited by ISQuA. 2016. Available online: http://nhsrcindia.org/index.php?option=com_content&view=article&id=171&Itemid=647 [Last accessed 2024 Sept 3]
- NABH Safe-I. 2015. Available online: http://nabh.co/safei.in/. [Last accessed 2024 Sept 3].
- Hospital infection control guidelines. 2016. Available online: http://icmr.nic.in/ guidelines/Hospital%20Infection%20control%20 guidelines- 2.pdf [Last accessed 2024 Sept 3].
- Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet. 2011;377:228-41.
- [CrossRef] [PubMed] [Google Scholar]
- Strengthening infection prevention and control and systematic surveillance of healthcare associated infections in India. BMJ. 2017;358:j3768.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pillars for prevention and control of healthcare-associated infections: An Italian expert opinion statement. Antimicrob Resist Infect Control. 2022;11:87.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Strategies to enhance rational use of antibiotics in hospital: A guideline by the german society for infectious diseases. Infection. 2016;44:395-439.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Selective and cascade reporting of antimicrobial susceptibility testing results and its impact on antimicrobial resistance surveillance-national healthcare safety network, April 2020 to March 2021. Microbiol Spectr. 2023;11:e0164622.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Selective and cascade reporting of antimicrobial susceptibility testing results and its impact on antimicrobial resistance surveillance-national healthcare safety network, April 2020 to March 2021. Microbiol Spectr. 2023;11:e0164622.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Selective and cascade reporting of antimicrobial susceptibility testing results and its impact on antimicrobial resistance surveillance-national healthcare safety network, April 2020 to March 2021. Microbiol Spectr. 2023;11:e0164622.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Analaysis and presentation of cumulative antimicrobial susceptibility test data. (M39-5th edition). CLSI; https://clsi.org/standards/products/microbiology/documents/m39/. [Last accessed 2024 Sept 3]
- Rapid diagnostic tests and antimicrobial stewardship programs for the management of bloodstream infection: what is their relative contribution to improving clinical outcomes? A systematic review and network meta-analysis. Clin Infect Dis. 2024;79:502-515.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antimicrobial stewardship: How the microbiology laboratory can right the ship. Clin Microbiol Rev. 2016;30:381-407.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mining the chemical space for new antibiotics. Nat Comput Sci. 2024;4:4.
- [CrossRef] [PubMed] [Google Scholar]
- WHO report: Landscape of diagnostics against antibacterial resistance, gaps and priorities. Available online: https://media.tghn.org/medialibrary/2021/04/WHO_diagnostics_report.pdf [Last accessed 2024 Sept 3]
- Tackling antimicrobial resistance by integrating One Health and the Sustainable Development Goals. BMC Global Public Health. 2023;1:11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Combating global antibiotic resistance: Emerging one health concerns in lower- and middle-income countries. Clin Infect Dis. 2018;66:963-969.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobials in agriculture and the environment: reducing unnecessary use and waste. London, UK: Review on Antimicrobial Resistance; 2015. Available at: http://amr-review.org/sites/default/oiles/Antimicrobials in agriculture and the environment - Reducing unnecessary use and waste.pdf. [Last accessed 2016 June 3]
- Antibiotic use and resistance in food animals: current policy and recommendations. Washington, DC: CDDEP; 2016. Available at: http://www.cddep.org/sites/default/oiles/india_abx_ report.pdf. [Last accessed 2017 Jan 5]
- Antibiotics overuse in animal agriculture: A call to action for health care providers. Am J Public Health. 2015;105:2409-10.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet Health. 2017;1:e316-e327.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Reducing veterinary drug residues in animal products: a review. Food Sci Anim Resour. 2019;39:687-703.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antibiotics use in food animal production: escalation of antimicrobial resistance: Where are we now in combating AMR? Med Sci (Basel). 2021;9:14.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The contribution of vaccination to global health: Past, present and future. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130433.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pollution from drug manufacturing: Review and perspectives. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130571.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Concentrations of antibiotics predicted to select for resistant bacteria: Proposed limits for environmental regulation. Environ Int. 2016;86:140-9.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic residues in the environment of South East Asia. BMJ. 2017;358:j2440.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Management options for reducing the release of antibiotics and antibiotic resistance genes to the environment. Environ Health Perspect. 2013;121:878-85.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bacterial population changes in hospital effluent treatment plant in central India. Water Res. 2004;38:441-7.
- [CrossRef] [PubMed] [Google Scholar]
- Multidrug-resistant Escherichia coli and Klebsiella pneumoniae isolated from hospital sewage olowing through community sewage system and discharging into the Indian Ocean. Bull Natl Res Cent. 2023;47:66.
- [Google Scholar]
- Antibiotics and antibiotic-resistant bacteria in waters associated with a hospital in Ujjain, India. BMC Public Health. 2010;10:414.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sewage effluent from an Indian hospital harbors novel carbapenemases and integron-borne antibiotic resistance genes. Microbiome. 2019;7:97.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]




