Translate this page into:
NAMS task force report on cervical cancer
*Corresponding author: Dr. Neerja Bhatla, Professor & Head, Department of Obstetrics & Gynaecology, All India Institute of Medical Sciences, New Delhi, India. Email: neerja.bhatla07@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhatla N. NAMS task force report on cervical cancer. Ann Natl Acad Med Sci (India) 2024;60:324-56. doi: 10.25259/ANAMS_TFR_11_2024
INTRODUCTION
Cervical cancer is the second most common cancer affecting Indian women. Two-thirds of women present with locally advanced disease despite it being a preventable cancer. Countries lacking an organized human papilloma virus (HPV) vaccination and screening program carry the major burden of the disease. Women in rural areas without adequate literacy and knowledge about cervical cancer are the ones who are the most affected. Despite efforts to combat cervical cancer, disparities in access to diagnosis and treatment persist. This highlights an urgency to address resource inequities in cancer care.
Cervical cancer is one of the few cancers which has a long premalignant phase and can thus be prevented by appropriate screening and timely clinical interventions. Various barriers, such as lack of manpower, infrastructure, and funding, as well as socioeconomic and cultural barriers have been impediments to the screening efforts. The national program for screening of common cancers (2016) proposed screening by visual inspection with acetic acid (VIA).1 However, to implement an effective, organized screening program in a large population, alignment with global standards is mandatory. Persistent infection with high-risk types of human papilloma virus (hr HPV) has been shown to be the necessary cause of cervical cancer. The World Health Organization (WHO) calls for the elimination of cervical cancer recommends HPV vaccination of 90% of girls under 15 years, screening by HPV test of 70% of women at 35 and again by 45 years, and treatment of 90% of lesions.2 The long-term promise of HPV vaccination is increasingly evident in countries that included the vaccine in the national program a decade ago.3,4 However, the immediate focus is needed for fortifying resources to diagnose and manage preinvasive and invasive cervical cancer cases. Addressing shortages in radiation and chemotherapy facilities, especially in smaller towns and rural regions, is pivotal for optimal cervical cancer care.
Raising awareness about cervical cancer, its causes, risk factors, and prevention is a cornerstone of the initiative. Public health campaigns should target communities, schools, workplaces, and media outlets to disseminate accurate information and dispel misconceptions. Government can play a pivotal role in policy formulation, funding allocation, and program implementation and collaboration among various stakeholders. This is imperative for the successful implementation of the initiative. A robust monitoring and evaluation system is required for tracking progress and identifying areas requiring improvement.
Challenges to be addressed include financial constraints, limited health care infrastructure, cultural barriers, vaccine hesitancy, and outreach to marginalized populations. The elimination initiative by WHO has the potential to save countless lives and significantly reduce the burden. By harnessing the collective efforts of governments, organizations, health care professionals, and communities, we can pave the way for the elimination of this preventable disease among our women and achieve the WHO targets in this regard by 2030.
The present report, under the auspices of the National Academy of Medical Sciences (NAMS), India, discusses the means and measures to address the problem of cervical cancer more coherently and effectively.
BACKGROUND
Medical professionals can play an important role in eliminating cervical cancer, the second most common cancer among women in India and a preventable one. The NAMS, India has taken the initiative by constituting a task force on cervical cancer with the objective of developing a white paper to be submitted to the Government of India for improving the health intervention activities in the area of cervical cancer. This white paper discusses the burden of cervical cancer in India and offers a roadmap for policymakers to address this issue more effectively with the help of medically oriented interventions. It will help various stakeholders to address the problem of cervical cancer in the Indian population.
OBJECTIVES
The main objectives of the task force are:
-
1.
To identify the current status in the area of cancer cervix.
-
2.
To identify the deficiencies which need to be addressed.
-
3.
To provide recommendations and future directions for making improvements in the field of cervical cancer.
METHODOLOGY
The task force members reviewed the published literature and data pertaining to cervical cancer in India. The initial working draft was circulated among the task force members, and comments were sought. Further modifications were made to the document based on the inputs received from the experts. They then developed a consensus on the key observations and recommendations, taking into consideration the health care services and the varied socioeconomic contexts across the Indian landscape.
CURRENT STATUS
Disease burden
India has a population of 511.4 million women aged 15 years and older who are at risk of developing cervical cancer, which accounts for 18.3% of all cancers5,6. It has been estimated that there were 127,526 newly diagnosed cases of cervical cancer and 79,906 reported deaths in 2022. Although the age-standardized incidence rate of cervical cancer has decreased substantially by 53.25% from 33.8 in 1990 to 18.0 in 2022, it is still the second most common cancer and a second most common cause of death due to cancer among Indian women [Figure 1a-1b and Table 1].6
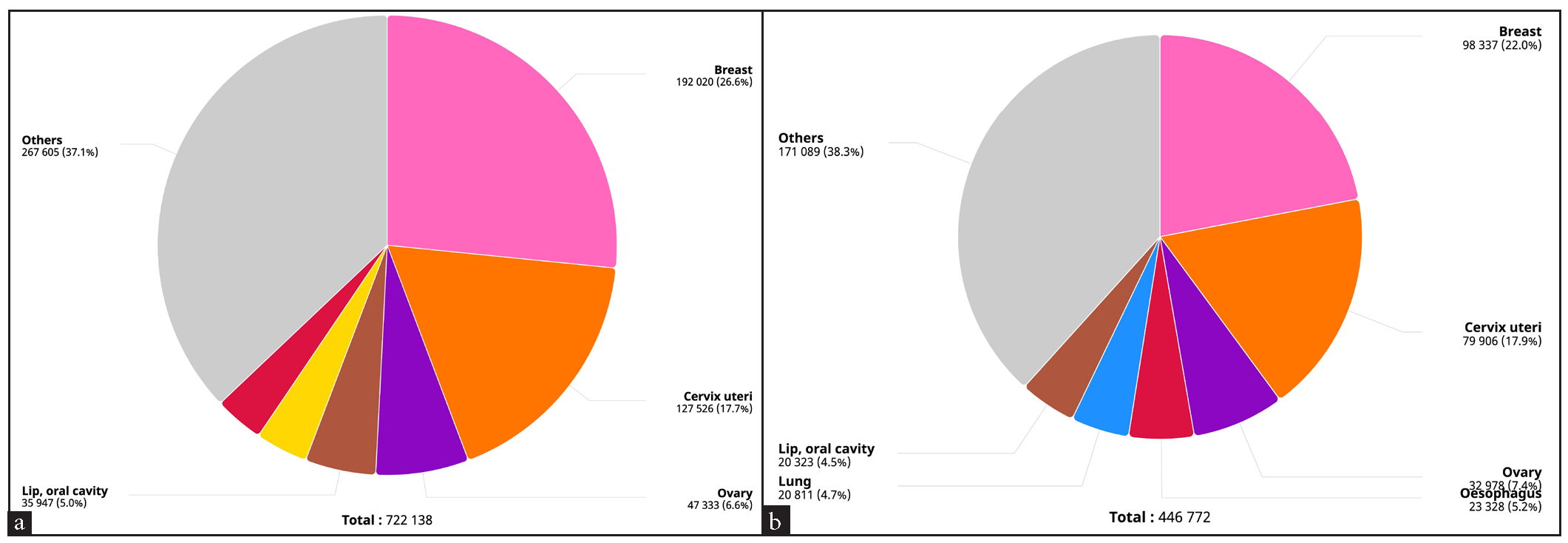
- Pie chart depicting annual incidence and mortality from cervical cancer in 2022. Source: Global Cancer Observatory (http://gco.iarc.fr).
| Incidence | Mortality | |
|---|---|---|
| Annual number of new cases/deaths | 127,526 | 79,906 |
| Crude rate | 18.9 | 11.8 |
| Age-standardized rate | 17.7 | 11.2 |
| Cumulative risk, 0–74 years (%) | 2.0 | 1.3 |
| Ranking of cervical cancer (all ages) | 2nd | 2nd |
| Ranking of cervical cancer (15–44 years) | 2nd | 2nd |
According to the report of the National Cancer Registry Programme (2012–2016) of the Indian Council of Medical Research, Papumpare district has the highest incidence rate of cervical cancer (27.7) in Asia [Figure 2a]. Cervical cancer is the leading type of cancer among women in Barshi Rural (AAR 15.3), Osmanabad and Beed (13.1), Mizoram (23.2), Tripura (9.8), Nagaland (9.3), Pasighat (20.3), and Cachar District (15.3). A significant decrease in the incidence rates has been observed in 10 population-based cancer registries (PBCRs), although an increase has been reported in some states [Figure 2b].7
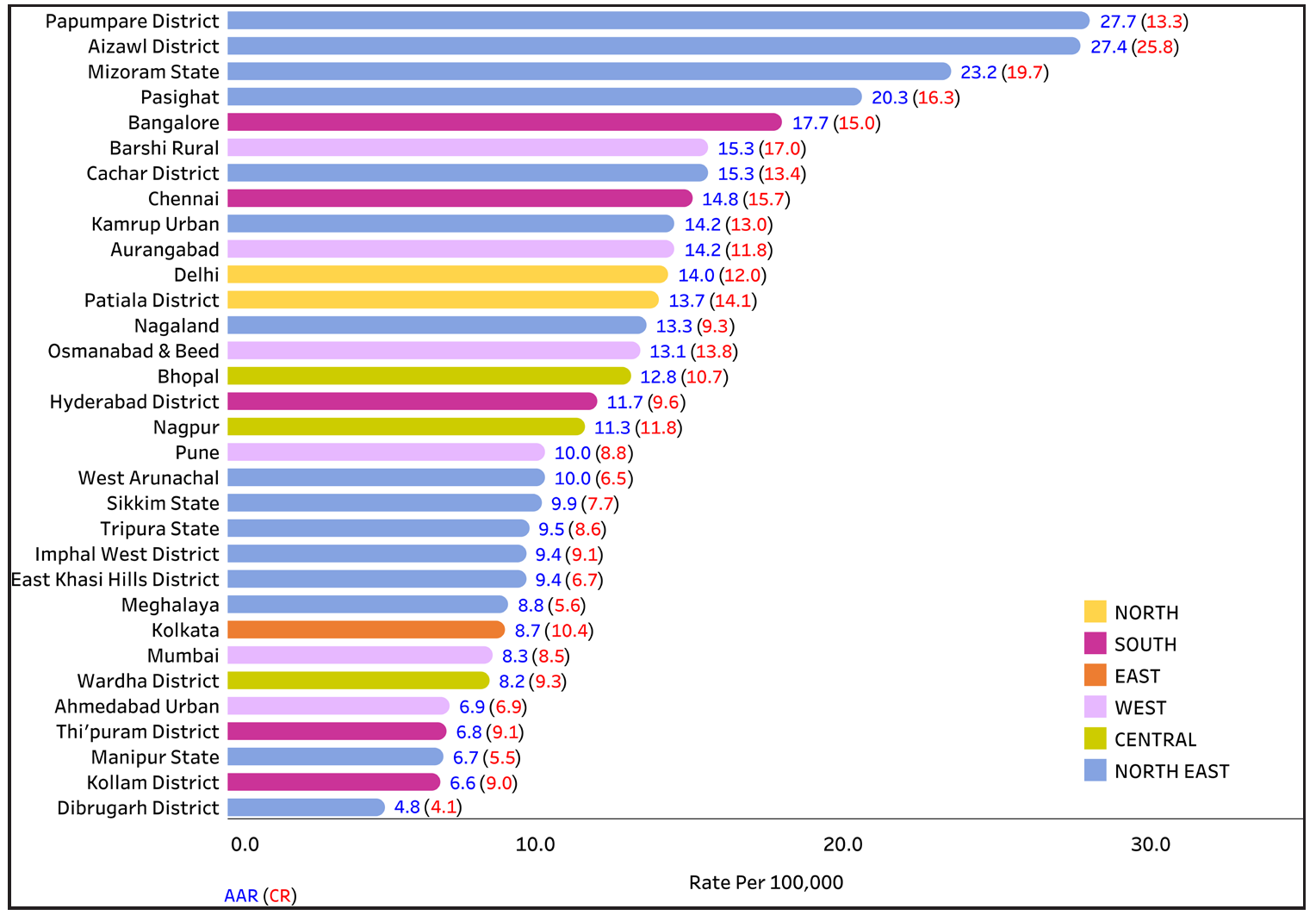
- Comparison of age-adjusted incidence rates (AARs) of 28 population-based cancer registries under the national cancer registry programme. Source: https://ncdirindia.org/All_Reports/ Report_2020/default.aspx.
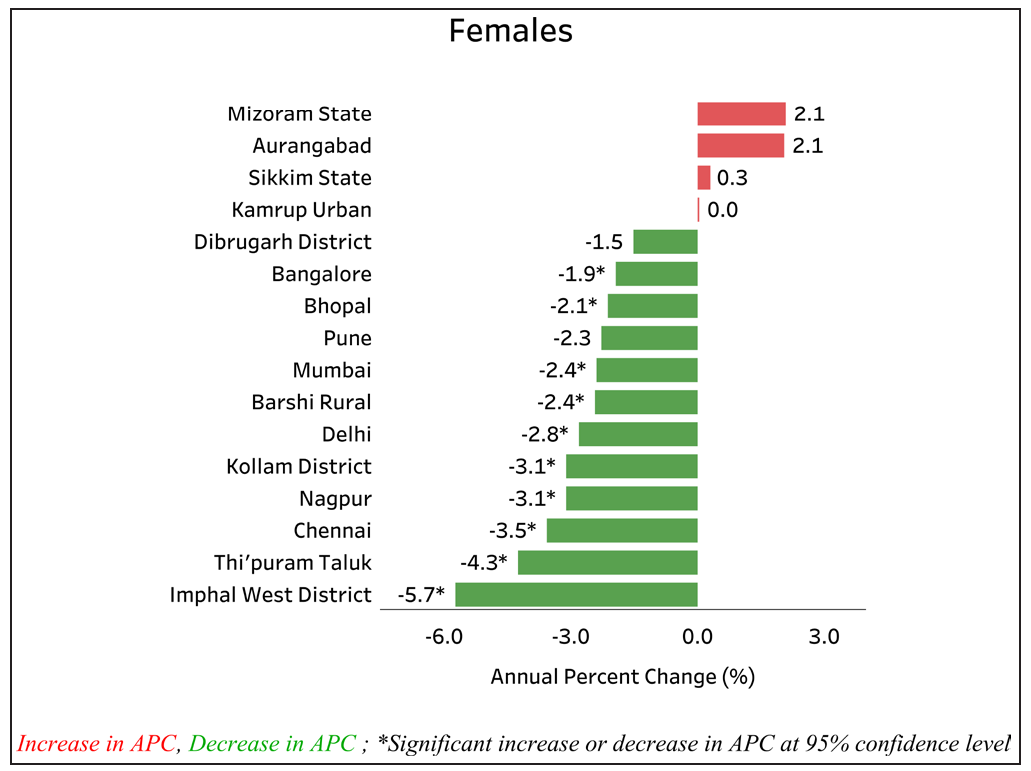
- Annual percent change (APC) in Age adjusted incidence rate (AAR) over the time period. Source: https://ncdirindia.org/All_Reports/ Report_2020/default.aspx.
Table 1 depicts the summary of the disease burden in India.
The median age at diagnosis is 50 years. It has been observed that the disease burden is negligible before the age of 30 years5 [Figure 3].

- Age-specific incidence rates of cervical cancer in India (estimates for 2020). Source: https://gco.iarc.fr/today.
Risk factors for cervical cancer and HPV genotypes in India
Risk factors
There are many known risk factors associated with the development of cervical cancer. Persistent hrHPV infection is the strongest risk factor, and it usually occurs in a background of other coexisting factors, as it is necessary but not sufficient. In India, about 5.0% of women in the general population harbor HPV 16/18 infection in the cervix at any given time, the two most common oncogenic types globally; 83.2% of all invasive cancers are HPV 16 or 18 positive, which is higher than the global average of 70%.5
The SES largely determines the risk of developing cancer cervix and plays a major role in survival too, as it is linked to multiple other risk factors. Approximately 85% of women with cervical cancer lives in a low middle-income country (LMIC). Directly related to the SES is the educational background. A population-based study conducted in south India showed that patients from a lower educational background have poor survival, and this was at least partially explained by having a more advanced disease at the time of diagnosis.8 Early age at marriage and onset of sexual activity, multiparity are other correlates linked with SES that are well-known risk factors in our population.9
Poor genital hygiene may be an indirect risk factor leading to genital infections, which can act as cofactors in the development of preinvasive lesions, according to a prospective study conducted in Kerala in 1999.10 Pelvic inflammatory disease due to various other factors, such as nutrition, immunity, and multiple sexual partners, increases the risk of cervical cancer. Coexistence of Chlamydia trachomatis and HPV 16 can increase the risk of cervical cancer.11
Tobacco smoking and coinfection with Human Immuno-deficiency Virus (HIV) have been identified as established cofactors, whereas herpes simplex virus type-2 (HSV-2), immunodeficiency, and certain dietary deficiencies are other probable cofactors.12
Prolonged use of oral contraceptive (OC) pills is a risk factor for cervical cancer. The relative risk in current users increased with increasing duration of OC use: use of OCs for 5 years can double the risk of cancer.13
Lack of awareness among women about the signs and symptoms of cervical cancer adds to improper utilization of screening services. In a knowledge, attitude, and practices (KAP) survey done in South India, the common symptoms of cancer cervix, such as intermenstrual bleeding and foul-smelling discharge, were reported by only a third of the patients. Similarly, the association of younger age at coitarche and marriage, as well as the increased risk with multiple sexual partners leading to repeated HPV infections, was known to only about a fifth of the population interviewed.14.
The first rural cancer registry was set up in 1987 at Barshi with a population of 0.4 million in western Maharashtra. Apart from the usual registry methodology, there was regular community interaction to educate on the warning signs of cervical cancer and motivate individuals to seek early medical attention. To overcome the adverse conditions in the rural areas, the registry adopted case finding in the community itself. The registry investigators visited the villages at least twice a year to identify the cases. Screening clinics were also set up in villages. The registry activity increased awareness in the population (P<0.01), increased the frequency of early cervical cancers by more than twofold during the past 16 years, and significantly decreased the relative risk of death (HR 0.7 [0.5–0.9]). According to the latest National Cancer Registry Programme (NCRP) data, there has been a significant decrease in age adjusted rate (AAR) over the time period, although carcinoma cervix still continues to be the leading cause of cancer among women in Barshi. This emphasizes the role of community awareness and education apart from the ongoing risk factors to be an important unmet area.15
HPV genotypes in India
In a meta-analysis including nine studies from India, the overall HPV prevalence was 12.0% in women with normal cytology/histology. The reported HPV-16/18 positivity was 78.9% in women with invasive cancer (87.7% in North and 77.2% in South India), 61.5% with high grade squamous intraepithelial lesion (HSIL), and 30.8% with low grade squamous intraepithelial lesion (LSIL). There was no difference in the overall HPV prevalence in cervical cancer between North and South India (P=0.063). However, HPV-16 and HPV-45 appeared to be more prevalent in North India (P=0.018 and 0.013, respectively), and HPV-35 in South India (P=0.033). Various high-risk HPV genotypes found among Indian women included types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59(16,17).
Worldwide, HPV types 16 and 18 are responsible for about 70% of all cervical cancer cases. In India, these two types are found in 83.2% of cervical cancers and in 28.2% and 62.8% of low-grade cervical lesions (LSIL/cervical intraepithelial neoplasia (CIN1)) and high-grade cervical lesions (HSIL/CIN2/CIN3/CIS), respectively. Other high-risk types categorized as probable/possible carcinogenic types are HPV 26, 30, 34, 53, 66, 67, 68, 69, 70, 73, 82, 85, and 97.5
In the latest report by the Catalan Institute of Oncology/International Agency for Research on Cancer based on various Indian studies, among 511.4 million women at risk for cervical cancer, about 5.0% are estimated to harbor cervical HPV-16/18 infection at a given time.5
Current status: HPV vaccination and screening
HPV vaccination and cervical cancer control
Vaccines against HPV genotypes 16/18 have been available since 2006 and have been recommended by WHO since 2009. Quadrivalent vaccines include low-risk HPV genotypes 6/11 as well. Since 2018, a nonavalent vaccine has been introduced that targets five additional hrHPVs, namely, 31/33/45/52/58.
HPV vaccines have been progressively introduced in many national immunization schedules and are presently included in the program in 130 countries. However, several studies and international agencies have reported that both vaccine introduction and coverage achieved are still suboptimal. In 2016, it was estimated that HPV immunization programs targeted only 12% of young adolescent females worldwide, and only 6% of girls aged 10–20 years had been vaccinated by end of 2014.18 The National Technical Advisory Group on Immunization (NTAGI) has recommended the inclusion of HPV vaccine in the Indian national program.
Given the highly effective and cost-effective prevention strategies available and the growing inequalities worldwide, WHO proposed a cervical cancer elimination strategy that includes scale-up of HPV vaccination to 90% of adolescent girls by 2030. This target is aligned with the Immunization Agenda 2030 and the sustainable development goals (SDGs) agenda (SDGs 3.4 and 3.b.1). HPV vaccination is the most cost-effective strategy to prevent cervical cancer in LMICs.19 Vaccination, screening, and treatment of preinvasive lesions are the pillars of WHO’s cervical cancer elimination strategy.
HPV vaccines currently available in India
-
1)
Gardasil [Merck Sharp & Dohme (MSD)] – Quadrivalent HPV vaccine, licensed in India in 2008, targets four HPV genotypes, high-risk types16 and 18, and low-risk types 6 and 11.
-
2)
Gardasil 9 (MSD) – Nonavalent HPV vaccine, licensed in India in 2022, targets nine HPV types, including high-risk types HPV 16/18/31/33/45/52/58 and low-risk types 6/11.
-
3)
Cervavac [Serum Institute of India (SIIL)] – Quadrivalent indigenous HPV vaccine, licensed in 2023, targets four HPV genotypes, high-risk types16 and 18, and low-risk types 6 and 11.
-
4)
The bivalent vaccine Cervarix Glaxo Smith Kline (GSK) for HPV 16/18 is presently not available.
HPV vaccine recommendations and efficacy
Dosage recommendations of various professional organizations and committees have been revised from time to time based on emerging information from various large studies and trials. Thus, there is a dichotomy between the vaccine dose recommendations on the product literature as licensed and that which is recommended for current practice.
The original guidelines for three-dose schedules at all ages were changed to two-dose guidance by WHO in 2018 for girls aged 9–14 years. In its latest position paper published in December 2022,20 WHO now recommends the following:
A one- or two-dose schedule for girls aged 9–14 years.
A one- or two-dose schedule for girls and women aged 15–20 years.
Two doses with a 6-month interval for women older than 21 years.
This has enormous implications, especially for LMICs, where there is a potential to improve coverage rates by increasing availability, decreasing costs, and improving logistics. The primary target of vaccination is girls aged 9–14, prior to the start of sexual activity. The minimum interval between the first and second dose should be 6 months. Immunocompromised individuals should receive at a minimum two doses and where possible three doses.
The vaccination of secondary targets such as boys and older females is recommended where feasible and affordable. Previously, there were shortages in the global HPV vaccine supply, but with the increasing availability of new vaccines and improved capacity of older vaccines, this is now set to change.
In June 2022, the NTAGI recommended the introduction of HPV vaccine in the Universal Immunization Programme in India with “a one-time catch-up for 9- to 14-year-old adolescent girls followed by routine introduction at 9 years”. This was based on the Indian evidence on the effectiveness of a single dose of HPV vaccine. In the India IARC trial, a multicenter, prospective, cohort study on vaccine efficacy against persistent HPV 16/18 infection at 10 years, after one, two, and three doses of quadrivalent HPV vaccine in girls, a single dose of HPV vaccine was found to provide similar protection against persistent infection from HPV 16 and 18, to that provided by two or three doses.21
Evolution of Indian data, the India IARC trial, and evidence leading to the recommendation for a single dose of HPV vaccine
In a cluster-randomized trial initiated in 2009, the investigators originally aimed to compare the immunogenicity, frequency of persistent HPV infection, and cervical precancerous lesions caused by vaccine-targeted HPV types after vaccination with two doses of quadrivalent vaccine on days 1 and 180 compared with three doses on days 1, 60, and 180. Suspension of recruitment and vaccination in 2010 due to events unrelated to the study led to some vaccinated girls receiving fewer than the planned number of vaccinations by default. As a result, the authors reanalyzed the data as an observational cohort study. The primary outcomes were immunogenicity in terms of L1 genotype-specific binding antibody titers, neutralizing antibody titers, antibody avidity after vaccination for the vaccine-targeted HPV types 16, 18, 6, and 11, and incident and persistent infections with these HPVs. Analysis was per actual number of vaccine doses received.
Of the 21,258 eligible girls in 188 clusters, 17,729 girls were recruited from 178 clusters before suspension. Four thousand three hundred and forty eight (25%) girls received three doses, 4979 (28%) received two doses on days 1 and 180 or later, 3452 (19%) received two doses on days 1 and 60, and 4950 (28%) received one dose. Immune response in the two-dose HPV vaccine group was noninferior to the three-dose group (median fluorescence intensity ratio for HPV 16 was 1.12 [95% CI 1.02–1.23] and for HPV 18 was 1.04 [0.92–1.19]) at 7 months, but was inferior in the two-dose default (0.33 [0.29–0.38] for HPV 16 and 0.51 [0.43–0.59] for HPV 18) and one-dose default (0.09 [0.08–0.11] for HPV 16 and 0.12 [0.10–0.14] for HPV 18) groups at 18 months. The geometric mean avidity indices after fewer than three doses by design or default were noninferior to those after three doses of vaccine. Fewer than three doses by design and default induced detectable concentrations of neutralizing antibodies to all four vaccine-targeted HPV types, though at lower concentrations after one dose.
Cervical samples from 2649 participants were tested; the frequency of incident HPV 16, 18, 6, and 11 infections was similar irrespective of the number of vaccine doses received. The testing of at least two samples from 838 participants showed that there were no persistent HPV 16 or 18 infections in any study group at a median follow-up of 4.7 years (IQR 4.2–5.1). Hence, it was concluded that the short-term protection afforded by one dose of HPV vaccine against persistent infection with HPV 16, 18, 6, and 11 is similar to that afforded by two or three doses of vaccine and required further assessment.22
In addition, the authors proposed that the two-dose recommendation of HPV vaccine could be expanded to girls aged between 15 and 18 years to reduce program cost and improve compliance. This was based on the subgroup analysis of 1795 girls aged 15–18 years receiving two (1–180 days) and 1515 girls of the same age receiving three (1-60-180 days) doses. Immunogenicity outcomes in 15- to 18-year-old two-dose recipients were also compared with the 10- to 14-year-old three-dose (N = 2833) and two-dose (N = 3184) recipients. At seven months, the 15- to 18-year-old two-dose recipients had noninferior L1-binding antibody titers against vaccine-targeted HPV types compared to three-dose recipients at 15–18 years and at 10–14 years of age. Neutralizing antibody titers at 18 months in 15- to 18-year-old two-dose recipients was noninferior to the same age three-dose recipients for all except HPV 18. The frequency of incident infections from vaccine-targeted HPV types in the 15- to 18-year-old two-dose recipients was similar to the three-dose recipients.23
Subsequently, the WHO recommendation has supported off-label single dose of HPV vaccine to reduce programmatic costs, mitigate supply shortages, simplify logistics, and allow more LMICs to introduce the vaccine. Hence, the durability of protection offered by a single dose becomes extremely important. In this respect, the authors conducted a study to determine whether single-dose recipients had sustained immune response against targeted HPV types at 10 years post-vaccination and whether this response was superior to the natural antibody titers observed in unvaccinated women. The antibody response observed over 120 months showed stabilized levels 18 months after vaccination for all four HPV types. Although the HPV type-specific (binding or neutralizing) antibody titers after a single dose were significantly inferior to those after three doses of the vaccine [lower bounds of geometric mean titer (GMT) ratios < 0.5], they were all significantly higher than those observed in unvaccinated women following natural infections (GMT ratios: 2.05 to 4.04-fold higher). Hence, a durable immune response in single-dose recipients of HPV vaccine at 10 years post-vaccination was confirmed.24
HPV vaccination coverage
In 2016, a multidisciplinary expert group constituted by the Secretary, Department of Health Research and the Director-General, Indian Council of Medical Research (ICMR) reviewed the available evidence globally regarding immunogenicity and efficacy, adverse effects, cost-effectiveness of the HPV vaccines and recommendations of WHO for the introduction of HPV vaccine at the country level. The group recommended that adolescent girls aged 9–13 years should be vaccinated with two doses of the HPV vaccine.25
Following this, an HPV vaccination program for school girls was launched in New Delhi on National Cancer Awareness Day (November 7, 2016), which vaccinated nearly 1200 girls. Simultaneously, the Government of Punjab initiated a well-planned campaign in two districts, vaccinating girls of Class 6 with 98% and 99% coverage in phase 1 and 2, respectively. In 2018, Sikkim became the first state to launch a state-wide program in which 25,284 school girls aged 9–14 years were vaccinated with 97% coverage.
In an evidence‐based impact projection study, HPV transmission model (EpiMetHeos) was adapted to current Indian data on sexual behavior, HPV prevalence, and cervical cancer incidence; assuming a 90% vaccination coverage in girls aged 10 years, HPV vaccination could effectively reduce the prevalence of HPV16/18 infection by 97% in 50 years with the age‐standardized incidence rate falling below the threshold for the elimination of 4 per 100,000 women years. This study also concluded that in girls aged 11–20 years, single‐dose vaccination along with catch‐up was more protective than two‐dose vaccination without any catch‐up, resulting in a decrease of 39%–65% versus 38% in lifetime risk of cervical cancer.26
At present, cost issues have played a major role in limiting the outreach of the available vaccines. Serum Institute of India Pvt. Limited (SIIPL) has developed and tested an indigenous quadrivalent vaccine, Cervavac, which will be affordable and is likely to be included in the national immunization program in the coming years.
Status of cervical screening
There are presently three accepted modalities of screening, namely, cytology, HPV testing, and VIA. While cytology has been the oldest method of cervical screening, established in the 1940s in the developed world, it has been seen to be effective only when performed with good-quality assurance and with repeated rounds of screening, as it has relatively poor sensitivity of about 55%.27 Its greatest strength lies in a high specificity which makes it better as a triage tool. Its widespread use is limited by the lack of resources in terms of laboratories and trained personnel. In India, it is available in cities and larger hospitals and medical colleges but, even there, there are limitations to the numbers that can be done. In a recent cross-sectional multicentric study conducted at tertiary care institutes across India, among the eligible women only 24.8% received screening. Availability of screening kits was limited to 10–25 Pap/HPV tests per day. VIA and HPV testing were offered only at certain centers. Colposcopy and treatment facilities were optimal at all centers (data under publication).28
VIA has sensitivity comparable to Pap smear but poorer specificity. It has the advantage of immediate results and the ability to be incorporated into a screen-and-treat program. While this makes it a suitable screening method for LMICs, the high false-positive rate means many women will be referred unnecessarily for triage, or be over treated in a screen-and-treat program. Repeated rounds of training for quality control, as well as linkages to secondary level facilities are necessary for scaling up coverage and adequate treatment.
HPV tests have the highest sensitivity with reasonable specificity and are presently the preferred choice for screening. They also have the best negative predictive value. This is the basis for the WHO recommendation to transition to HPV tests with a goal of screening 70% twice in their lifetime, by age 35 and again by age 45 years. However, it is essential to have validated tests with quality control; otherwise, there will be a large number of false positives and negatives.29 Point-of-care HPV tests will be useful in the screen-and-treat strategy. Self-sampling is emerging as the sampling type of choice under WHO’s recommended self-care guidelines. While both DNA and RNA tests are being used in screening, self-sampling is presently recommended for HPV DNA tests only. Tests are also available that have partial HPV genotyping which works as an inbuilt triage. HPV-positive cases that are HPV 16/18 positive can be considered for a screen-and-treat approach.
For HIV-positive women, the WHO recommends using HPV DNA as the primary screening test rather than VIA or cytology, along with triage after a positive screen. In these women, screening should start at 25 years and the recommended screening interval is 3–5 years.30
Linkage of screening with treatment is essential to prevent cervical cancer. A screen and treat approach has been recommended by WHO. For HIV-positive women, the screen, triage, and treat approach is recommended.
In 2016, MoHFW released the Operational Framework for the Management of Common Cancers under the National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases & Stroke (NPCDCS), renamed National Programme for Non Communicable Diseases (NP-NCD) in 2023.1 VIA was implemented as the test of choice for screening women between 30 and 65 years of age, with specific guidelines for the screen-and-treat approach. However, several barriers have been observed in implementation. A pilot study conducted at Silchar, Assam, in 2018 found a lack of human resources, overburdening of the existing staff, and difficulty in motivating the community for screening as the top three challenges in implementation.31
The National Family Health Survey (NFHS-4) 2015–2016 reported that 22% of women have undergone cervical screening in India, and the majority of the districts fall in the range of 10–20% coverage. As per the 2021 India factsheet of WHO, the coverage of cervical cancer screening is only 3.1%32,33. Another study conducted in 2020 in South India revealed that only 14.3% had at least one-lifetime pelvic exam, and 7.1% had undergone cervical cancer screening.34 The higher percentage reported here maybe due to the fact that responders may have perceived speculum exam or even a pelvic exam of any sort, most likely related to antenatal and pregnancy care, as cervical cancer screening. The recent NFHS-5 data are also in line with the WHO data, where the percentage of women who have ever undergone cervical cancer screening in India is 1.9% (2.2% in urban areas and 1.7% in rural areas) [Figure 4].
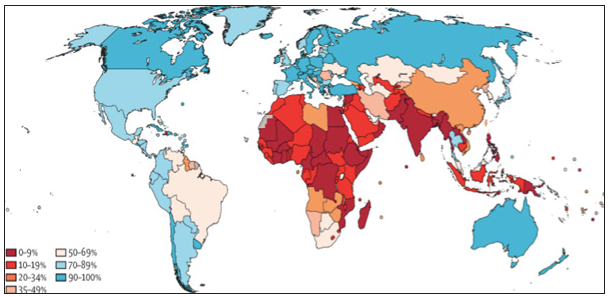
- Countrywise ever in lifetime cervical cancer screening coverage in women aged 30–49 years. Source: https://hpvcentre.net
Andhra Pradesh, Bihar, Jammu and Kashmir, Telangana, and West Bengal have more rural women participating in cervical cancer screening than those in urban areas. The women living in the urban regions of Mizoram, Himachal Pradesh, Kerala, and Maharashtra have a significant number of women undergoing a screening test for cervical cancer. The practice of cervical cancer screening is close to insignificant in Nagaland, Ladakh, and Gujarat.35
The Tamil Nadu government conducted successful pilot programs and subsequently rolled out a cost-effective and operationally feasible large-scale cancer screening program. The pilot project was started in Chennai Corporation in 2005, and scaled to a district-level pilot in February 2007 by the World Bank that supported the Tamil Nadu Health Systems Project. State-wise scaling up in 16 districts in 2012 was later extended to the remaining 16 districts in 2013.36 The main components included a cost-effective VIA-based screening strategy with a screen-and-treat approach, mass awareness campaigns, self-help groups to reach the community, trained personnel, diagnostic and treatment services at all levels with assured linkage between the facility centers, interdepartmental coordination with school education and labor welfare departments, data analysis, quality assurance with intensive monitoring, and supervision and online reporting system by the health management information system. By 2016, 81% of the target population was screened with 3.3% positivity rate. However, the positivity rates, compliance with colposcopy, and CIN detection rates were far lower than had been seen in a cluster-randomized trial in Tamil Nadu by IARC, in which VIA-based screening showed a reduction in incidence by 25% and mortality by 35% over a follow-up period of 7 years.37 In another cluster-randomized study conducted in Mumbai to investigate the efficacy of VIA performed by primary health workers in reducing cervical cancer mortality showed a 31% reduction.38 However VIA screening, requires good training and sustained quality assurance to be an effective method to prevent cervical cancer in developing nations.
The poor specificity of VIA (53.3%) is a major drawback; evidence suggested the use of adjunctive tests like addition of HPV testing to VIA to increase its specificity (95.4%). This approach had the potential to reduce referral rates without compromising the sensitivity.39 Subsequent studies suggested that a single round of HPV testing may be a more effective strategy in reducing the incidence and mortality. In the cluster-randomized trial conducted in rural India to measure the effect of a single round of screening by testing for HPV, cytologic testing, or VIA, authors found a significant reduction in the numbers of advanced cervical cancers and deaths by using a single round of HPV testing.40 Another study to evaluate the effectiveness of VIA, Pap, and HPV testing in a cervical cancer screening program in a periurban community in Andhra Pradesh concluded that HPV testing had higher sensitivity (100%) and specificity (90.6%) compared to cytology (sensitivity=78.2%; specificity=86.0%) and VIA (sensitivity=31.6%; specificity=87.5%). The authors also suggested that potentially 87.6% of underlying cases of CIN3 and cancer may have been missed due to program failure.41
HPV testing has been recommended by WHO as the primary screening modality, and the development of rapid, point of care HPV tests along with the choice of self-sampling has the potential to make it the future screening modality. A cross-sectional study to examine the concordance between HPV by Hybrid Capture 2 (HC2) and polymerase chain reaction (PCR) on self-collected vaginal and physician-collected cervical samples showed that the concordance between HC2 and PCR was 90.9% for self-samples (kappa=63.7%, 95% CI: 55.2–72.2%) and 95.3% for physician-collected samples (kappa=80.4%, 95% CI: 71.8–89.0%).42
Presently, indigenous HPV tests have been developed and efforts are ongoing for validation by international standards so that these can be included in the national program with confidence.
Status of surgical facilities in the country
Surgery for cervical cancer
The type and extent of surgery for cervical cancer is determined based on the FIGO stage of the tumor – the size, histological type and extent of tumor, desire for future fertility, and any comorbidities. Table 2 shows the various surgical procedures that may be performed for the management of patients with cervical cancer.
| Procedure | Indications |
|---|---|
| Large loop excision of the transformation zone (LLETZ), also known as loop electrosurgical excision procedure (LEEP) | Diagnostic procedure; treatment of high-grade intraepithelial neoplasia (CIN 2–3) |
| Cold knife conization | Diagnostic procedure; fertility-sparing procedure in stage IA1 disease |
| Type B and C1 (nerve-sparing) radical hysterectomy + pelvic lymph node dissection/sentinel lymph node biopsy | Stage IA2–IB1 |
| Type C2 radical hysterectomy + pelvic lymph node dissection | Stage IB2 |
| Radical trachelectomy + pelvic lymph node dissection/sentinel lymph node biopsy | Stage IA2-IB1, fertility-sparing procedure |
| Ovarian transposition | Ovarian function preservation prior to pelvic radiation in young patients |
| Pelvic exenteration | In select patients with stage IVA/recurrent disease |
| Urinary/bowel diversion procedures | Palliative procedures |
Radical surgery is the preferred treatment modality for early stage cervical cancer. However, due to ack of a population-based screening program in the country, only a small proportion (generally less than 10%) of women with cervical cancer present in the early stage and are candidates for radical surgical resection. However, this is expected to change with increasing implementation of screening programs. Open abdominal route is the current standard of care for radical hysterectomy for cervical cancer. In a randomized controlled trial of open versus minimally invasive surgery (MIS) for cervical cancer, the rate of disease-free survival (DFS) at 4.5 years was 86.0% with MIS and 96.5% with open surgery. The hazard ratio for disease recurrence or death from cervical cancer in the MIS group was 3.74 (95% CI 1.63–8.58), a significant difference that remained after adjustment for age, body mass index, stage of disease, lymphovascular invasion, and lymph node involvement. MIS was also associated with a lower rate of overall survival (OS) (3-year rate, 93.8% vs. 99.0%; hazard ratio for death from any cause, 6.00; 95% CI, 1.77–20.30). A higher proportion of vault recurrences occurred in the open surgery group (43%, as compared with 15% in the MIS group), and all nonvaginal vault recurrences occurred in the MIS group. This was a new pattern of recurrences in the peritoneal cavity seen only in the MIS group and subsequently confirmed by several other reports as well.43 Subsequent to these findings, MIS is contraindicated due to poorer oncological outcomes compared to the open surgical route and should not be offered outside a clinical trial setting.
The hypothesis to downsize the tumor in patients with locally advanced cervical cancer (LACC) by the use of neoadjuvant chemotherapy (NACT) to make the disease amenable to radical surgery has fascinated researchers for decades and has been investigated in two large trials44,45. A phase III randomized controlled trial was conducted at Tata Memorial Centre, Mumbai to evaluate the role of NACT followed by radical hysterectomy in patients with LACC. Six hundred and thirty-five patients with International Federation of Gynecology and Obstetrics (FIGO) stage (2009) IB2, IIA and IIB, squamous cell carcinoma of the cervix were randomized to NACT-surgery ± adjuvant treatment or concurrent chemoradiation. Results of this trial showed inferior DFS with the NACT surgery compared to the standard concurrent chemoradiation. At a median follow-up of 58.5 months, five-year DFS was 69.3% in NACT-surgery and 76.7% in the chemoradiation arm (P=0.03). There was no difference in OS between the two treatment groups. Similar results are shown in a recently published, multicenter trial conducted by European Organisation for Research and Treatment of Cancer (EORTC). Results of these two large trials do not support the use of NACT and radical surgery in patients with LACC, and concurrent chemoradiation remains the standard of care for these patients. Currently, radical surgery is recommended only in patients with low-risk FIGO 2018 stage IB1-2 and stage IIA1 disease.46 The likelihood of the need for adjuvant radiation in more advanced tumors increases the morbidity from combined modality therapy as well as places additional burden on the health system.
Evolution of gynecologic oncology specialty in India and its current status
The recognition of the subspecialty of gynecologic oncology as an independent discipline in India is relatively recent. In the year 2011, the Medical Council of India (MCI) approved the Magister Chirurgiae (MCh) in gynecologic oncology as a 3-year comprehensive training program. The course was first started at the Tata Memorial Hospital (TMH), Mumbai, with a single student per year. As a result of persistent, proactive actions, both from various academic centers and regulatory bodies, within a span of 12 years, there has been an exponential increase in the number of seats and training centers across the country; currently, 11 centers provide training opportunities to 28 students per year [Table 3].
| S. No. | Name of Institute, City | No. of Seats Per Year |
|---|---|---|
| 1 | Acharya Harihar Regional Cancer Centre, Cuttack | 2 |
| 2 | All India Institute of Medical Sciences, New Delhi | 5 |
| 3 | AIIMS, Rishikesh | 2 |
| 4 | Amrita Institute of Medical Sciences, Kochi | 2 |
| 5 | Christian Medical College, Vellore | 3 |
| 6 | Dr. Bhubaneshwar Borooah Cancer Institute, Guwahati | 2 |
| 7 | Gujarat Cancer & Research Institute, Ahmedabad | 4 |
| 8 | Kidwai Memorial Institute of Oncology, Bengaluru | 3 |
| 9 | Regional Cancer Centre, Thiruvananthapuram | 2 |
| 10 | St. John’s Medical College, Bengaluru | 1 |
| 11 | Tata Memorial Centre, Mumbai | 2 |
Further, augmentation to the subspecialty of gynecologic oncology occurred with the approval of Doctorate of National Board (DrNB), and Gynaecologic Oncology by the National Board of Examination (NBE) in 2019. At present, 16 students per year across 11 centers can enroll in this course [Table 4].
| S. No. | Name of Institute, City | No. of Seats Per Year |
|---|---|---|
| 1 | Apollo Hospitals, Bannerghatta Road, Bengaluru | 1 |
| 2 | Chittaranjan National Cancer Institute, Kolkata | 2 |
| 3 | Dharamshila Narayana Superspeciality Hospital, Delhi | 2 |
| 4 | Fortis Memorial Research Institute, Gurgaon | 1 |
| 5 | Lakeshore Hospital and Research Centre, Kochi | 1 |
| 6 | Mahavir Cancer Sansthan & Research Centre, Patna | 2 |
| 7 | Medanta, The Medicity, Gurgaon | 1 |
| 8 | Rajiv Gandhi Cancer Institute and Research Centre, Delhi | 1 |
| 9 | Sri Shankara Cancer Hospital and Research Centre, Bengaluru | 1 |
| 10 | Tata Medical Centre, Kolkata | 2 |
| 11 | Vardhaman Mahaveer College and Safdarjung Hospital, Delhi | 2 |
Admission to these courses is through superspecialty national entrance and eligibility tests (NEET), followed by an online counseling, conducted by the Directorate General of Health Services, New Delhi.
The ability to perform a radical hysterectomy and pelvic lymph node dissection defines a gynecologic oncologist. However, the optimum surgical management of a patient with cervical cancer requires not only surgical skills and training but also an understanding of disease biology, preoperative evaluation to assess suitability for surgery, a detailed knowledge of the surgical anatomy of the pelvis, management of perioperative complications, and postoperative adjuvant treatment planning. The above-mentioned training programs are conducted by academic centers with adequate clinical workload and infrastructure and a well-designed, structured curriculum. During training, students get to learn complex surgical skills as well as comprehensive multidisciplinary management of gynecological cancer patients, including basic principles and techniques of systemic therapy, radiation therapy (RT) and palliative care, and preventive gynecologic oncology. The development of surgical skills is a continuous process that evolves over several years. Continuous practice, mentorship, regular appraisal, and learning new surgical skills are ongoing processes throughout a surgeon’s professional career.
With the establishment of the subspecialty of gynecologic oncology in the last decade and the availability of trained gynecologic oncologists in many cities, the proportion of cervical cancer patients undergoing surgery by a gynecologic oncologist is steadily increasing. However, considering our huge population, heterogeneity, and wide disparities in health care resources and still limited availability of gynecologic oncologists in most parts of the country, a substantial proportion of patients with cervical cancer undergo surgeries by a nongynecologic oncologist; including general gynecologists, general surgeons, and surgical oncologists. The latter, during their training as surgical oncologists, undergo rotation in gynecologic oncology. However, evidence suggests that outcomes of gynecologic cancer patients are better when managed by specialists trained in gynecologic oncology compared to those managed by generalists.47,48
Besides MCh and DrNB training programs, there are several university-recognized fellowship courses offered by various cancer centers across the country. The Association of Gynaecologic Oncologists of India (AGOI) accredits gynecologic oncologists to conduct fellowship programs (http://www.agoi.org/educational-activities/fellowship).
Inappropriate surgical management of patients with cervical cancer in India
Despite clear guidelines on the indications of radical surgery for invasive cervical cancer, a considerable number of women still undergo inadequate or improper surgery in the country. The incidence of cervical cancer diagnosed after inadvertent simple hysterectomy has been reported to be 5–15%. In a retrospective analysis of 768 patients with cervical cancer presenting to the Tata Memorial Hospital from January to June 2019, 87 patients (11.3%) had inadequate surgery prior to presentation: simple abdominal hysterectomy in 77 patients (88.5%), vaginal hysterectomy in 5 patients (5.7%), and subtotal hysterectomy in 6 patients (6.9%). Forty-one patients (47.1%) had residual disease at presentation (unpublished data). Several factors have been identified to be responsible for inadequate/improper surgery including lack of routine screening for cervical cancer, inadequate diagnostic workup prior to surgery, limited availability of dedicated cancer centers equipped with surgical and radiation oncology facilities, deliberate hysterectomy for grossly invasive cancer, misreading of pathology results, errors at the colposcopic examination, etc.49 A study from Northeast India found that failure to perform preoperative Papanicolaou smear, incomplete evaluation of cervical intraepithelial neoplasia (CIN) on cervical biopsy, and negative Papanicolaou smear accounted for 75% of the patients undergoing inappropriate simple hysterectomy. The study also showed a significant delay in referral to an oncology center after inadvertent simple hysterectomy; 23% (12/52) patients were referred more than 100 days after hysterectomy.50 Inadequate or improper surgery adversely affect patients’ survival. Patients with residual or recurrent vaginal cancer after surgery for cervical cancer have modest outcomes with chemoradiation and with significant treatment-related toxicity.51,52
Improper surgery in patients with invasive cervical cancer can be avoided by implementation of universal screening for cervical cancer and optimum management of screen positives. A thorough preoperative evaluation of patients should be done before scheduling for a “benign hysterectomy.” If a gross cervical lesion is visualized, irrespective of the cytology report, a biopsy must be mandatory prior to hysterectomy, and timely referral to an oncology center with all clinical details and biopsy tissue should be made so that treatment can be started at the earliest. One of the key strategies to decrease the morbidity and mortality from cervical cancer is to further strengthen the subspecialty of gynecologic oncology.
Status of radiation facilities in the country
In India, the majority of patients present in locally advanced stages, where surgery plays a limited role. The specialty of RT has progressed rapidly over the past two decades with the development of more sophisticated planning and delivery techniques. The introduction of computer technology and imaging has galvanized the practice of RT, and advancement in RT techniques has yielded improved clinical outcomes with reduced toxicity.
RT can be used in different settings for the management of patients with cervical cancer: (i) as definitive therapy for curable patients, (ii) as adjuvant therapy for operated patients to prevent locoregional recurrence, and (iii) as palliative therapy for alleviating the distressing symptoms in patients with advanced incurable disease.
The radiotherapy centers in India have either teletherapy facilities alone or both teletherapy and brachytherapy facilities. Currently, India has approximately 704 teletherapy machines (Linear Accelerator 544, Telecobalt 160), 22 advanced therapy machines (7 Gamma knife units, 22 Tomotherapy machines, 10 Cyber knife machines, and 2 proton beam therapy centers). Every year, around 40 external beam therapy units are added and 15 units are decommissioned, bringing the total number of new units to 25 per year.
Brachytherapy remains an integral portion of the RT treatment of cervical cancer. It can be used in different settings, viz., intracavitary, interstitial, and combined intracavitary and interstitial. Cervical cancer patients treated without brachytherapy experience compromised survival outcomes. The modern high-dose rate (HDR) remote after-loading brachytherapy machines are gradually replacing low-dose rate (LDR) units as these have several advantages. Presently, the number of remote after-loading brachytherapy units in India is around 325. Of these, about 280 are HDR after-loading units, and around 50 are LDR units. It is emphasized here that every RT center must have brachytherapy services so as to impart comprehensive treatment to cervical cancer patients.
As per the Directory of Radiotherapy Centres (DIRAC) data, Western Europe and North America have more than three teletherapy machines per 1 million population, while India has less than 1 machine per 3 million population [Figure 5]. This is grossly inadequate as per the WHO recommendations.
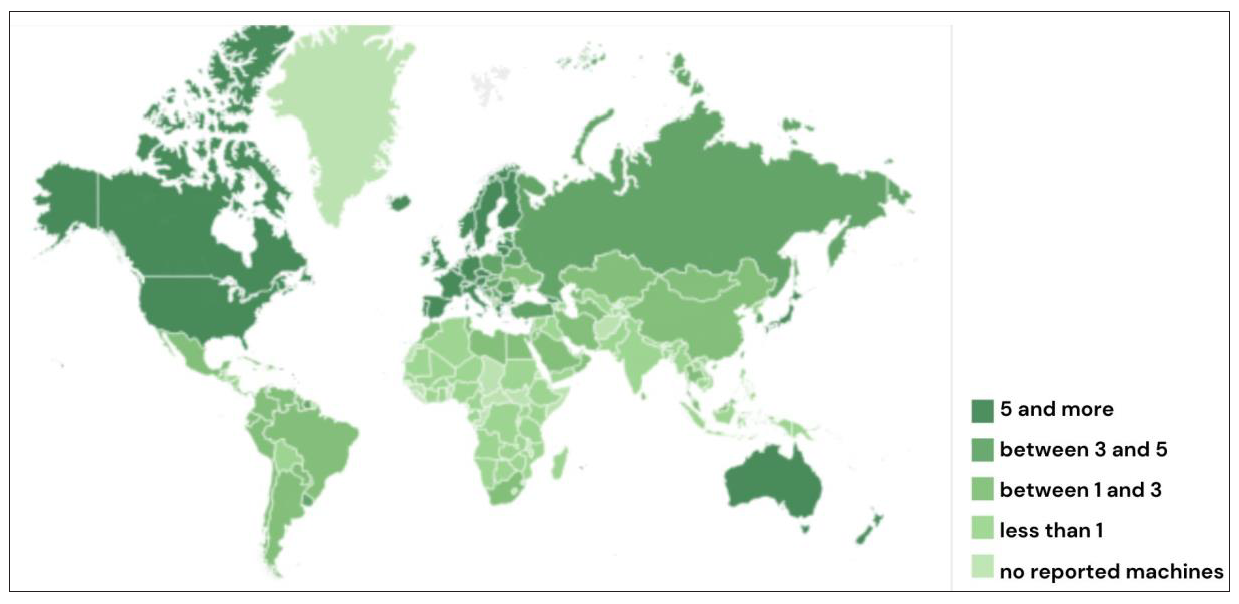
- Global map of radiotherapy machine distribution. Source: The IAEA Directory of Radiotherapy Centres (DIRAC) https://dirac.iaea.org. Accessed on Nov 9, 2024. IAEA: International Atomic Energy Agency
Apart from the gross inadequacy of RT facilities, the distribution of centers is also heterogeneous. Most of the RT centers are concentrated in large metropolitan cities like Delhi, Mumbai, Kolkata, Chennai, Bengaluru, and state capitals while rural areas with a major burden of cancer cervix do not have RT machines in their vicinity. This can be attributed to the factor that RT machine installation and usage requires technically trained staff as well as good infrastructure. Also, quality assurance is pivotal in running a RT center. In terms of brachytherapy equipment, the state of affairs is even more dismal [Figure 6]. Not every teletherapy center has a brachytherapy machine and even when a brachytherapy machine is available, centers are not able to utilize it optimally due to a lack of expertise and unavailability of accessories along with other logistic and regulatory issues.53
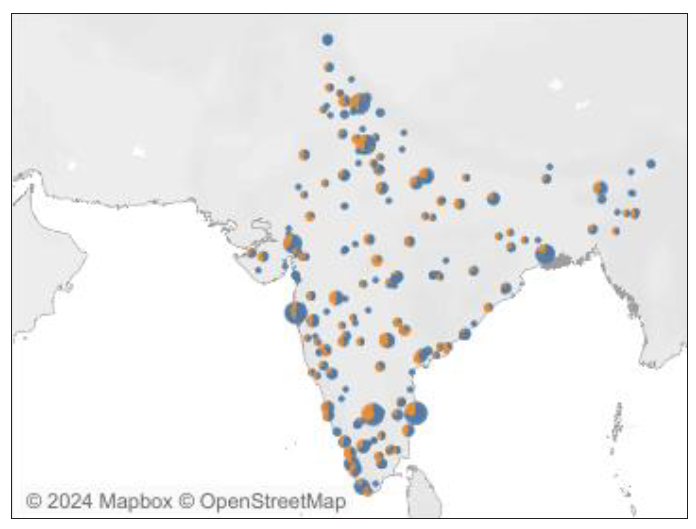
- Distribution of radiotherapy equipment in India. Source: The IAEA Directory of Radiotherapy Centres (DIRAC) https://dirac.iaea.org. Accessed on Nov 9, 2024. IAEA: International Atomic Energy Agency
Recent reports also suggest that the high costs incurred on travel to distant centers leads to noncompliance. A study performed at a rural cancer center indicated that over 60% of patients were noncompliant citing difficulties in travel. These patients had to travel a distance of more than 100 km from home to hospital.54
We need one RT machine per million population for adequate RT services. In its 2023 report, Directory of Radiotherapy Centres (DIRAC) has included India along with the poorest Sub-Saharan African countries.53 The health care policy in the future must include galvanizing more RT resources in order to meet the required infrastructure. Distribution of medical colleges are now at par across the Indian geography. But, not all medical colleges have a radiation oncology department. Population data of cancer incidence and prevalence are now available to enable decision-making; an initiative should be taken to earmark geographical areas where RT center along with other cancer treatment facilities can be installed.
Status of systemic therapy and gaps
Early-stage disease is managed through surgical approaches, while the standard of care for locally advanced yet nonmetastatic cases involves concurrent chemoradiation. Nonetheless, relapses remain common even with post-curative treatments. The incidence of relapse rises with advanced stages, with nodal positivity emerging as an independent adverse prognostic indicator. The -year survival rate for patients in stages IIIC and IVA dwindles to a mere 15–20%. In advanced stages, the majority of relapses manifest systemically, significantly constraining the success rate of salvage therapies. Systemic treatments, encompassing chemotherapy, targeted therapy, and immunotherapy, are employed for these cases. Despite these efforts, post-relapse treatment options are limited, resulting in many patients eventually succumbing to the disease.
Outcomes from NCRP data from 2012 to 2016 show that in 60.0% cases, the clinical extent of the disease was locoregional. Localized disease was seen in 32.8%, whereas distant metastases were observed in 5.1% cases. A high proportion of patients with cancer cervix uteri underwent chemotherapy plus radiation (localized 49.8%, locoregional 56.6%, distant metastases 46.7%, and stage unknown 38.3%). Radiotherapy alone was the second most preferred treatment for cervical cancer. Only 7.7% patients with clinically localized cancer cervix uteri were treated with surgery [Table 5].7 This highlights the need for strengthening the availability of systemic therapy options.
| Treatment | Localized only | Locoregional | Distant metastasis | Unknown |
|---|---|---|---|---|
| N% | N % | N % | N % | |
| Surgery | 592 7.7 | 318 2.3 | 10 0.8 | 41 7.9 |
| Radiotherapy (RT) | 1935 25.1 | 4356 30.8 | 442 37.1 | 172 33.1 |
| RT+chemotherapy | 3842 49.8 | 8005 56.6 | 556 46.7 | 199 38.3 |
| Systemic therapy | 340 4.4 | 689 4.9 | 133 11.2 | 57 11.0 |
| Multimodality* | 995 12.9 | 682 4.8 | 42 3.5 | 46 8.9 |
| Palliative care | 18 0.2 | 83 0.6 | 7 0.6 | 4 0.8 |
| Surgery | 592 7.7 | 318 2.3 | 10 0.8 | 41 7.9 |
| Total | 7722 100 | 14133 100 | 1190 100 | 519 100 |
Current systemic therapy options
For locally advanced cases: concurrent chemotherapy with cisplatin, coupled with radical radiotherapy is now the standard of care.
For metastatic and relapsed cases, the following options are in use:
-
1.
Chemotherapy: The standard first-line treatment typically involves paclitaxel and carboplatin. However, responses are usually short-lived, and progression is inevitable.
-
2.
Targeted Therapies: These include anti-angiogenic drugs such as bevacizumab, which, when combined with chemotherapy, have demonstrated improved survival rates in studies.55
-
3.
Immunotherapy: Recent advancements in immune therapeutics have led to the increased use of anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PDL-1) blockers as the standard of care in various cancers, including cervical cancer.56 While these drugs have shown promise, their use remains limited due to high costs.
Existing gaps
1. Locally Advanced Cervical Cancer
FIGO Stage IIIC and Stage IVA patients have a discouraging -year survival rate of 15–20%. A significant proportion of relapses in advanced stages manifest systemically, hampering the efficacy of salvage therapies. This highlights an unmet need to enhance outcomes within this subset of cervical cancer patients. Recent studies evaluating the addition of extra chemotherapy cycles to standard concurrent chemoradiation did not yield positive results. Therefore, there is a need for more rational and pragmatic trial designs to address this gap.
2. Recurrent and Metastatic Cases
Efforts should be directed toward researching less toxic therapies, such as exploring low-cost options like oral metronomic chemotherapy, evaluating low-dose immunotherapy, and facilitating the development of generic immunotherapies. Encouraging global pharmaceutical companies to expand compassionate access programs to Indian patients and conducting clinical trials in India would also be beneficial.
Collaborative efforts
The formation of cervical cancer specific research groups dedicated to novel preventive approaches and the promotion of research for developing low-cost, less toxic, and efficient therapies are necessary.
Inadequacies in Indian data underline the necessity for collaboration between academic institutions to foster data collection, aggregation, and analysis aimed at identifying specific issues.
Molecular research
Advancements in molecular research are needed that could potentially yield significant insights for developing newer effective therapies for cervical cancer management.
Current health programs and national guidelines on screening and management
Ministry of Health and Family Welfare (MoHFW) has taken numerous steps over the years to control this preventable cancer at the national level.
The National Cancer Control Programme (NCCP) was launched in 1976 with the aim of strengthening tertiary care institutions and to improve holistic care for cancer from prevention to palliation. Subsequently, in the late 1990s, the priorities were redefined, and the program aimed at primary and secondary prevention, which included health education, awareness about the disease, and screening using cytology (Pap smear) to prevent the disease. This was practically feasible at the ground level by the launch of the Modified District Cancer Control Programme (MDCCP).
In 2010, NCCP was integrated with the NPCDCS, renamed as National Programme for Non-Communicable Diseases (NP-NCD) in 2023. It was rolled out in 21 states, initially under noncommunicable disease (NCD) clinics in community health centers (CHC). Beyond this, there is the tertiary care cancer centers (TCCCs) scheme, the aim of which is to strengthen/set up state cancer institutes (SCI) and TCCCs to provide comprehensive cancer care. Under the NPCDCS program, VIA was recommended for all women between 30 and 59 years of age by healthcare workers, and protocols were made for the management/referral of VIA-positive cases for colposcopy and further treatment as and when required.1 Training of health care professionals on VIA/colposcopy and on ablative methods were also part of this program. In 2016–2018, MoHFW developed the mobile technology platform for cervical cancer screening, which helped in the implementation and continuous monitoring of the screening program in each state.
With the increasing need for cancer-screening guidelines in India, ICMR’s National Institute of Cancer Prevention and Research (NICPR), Noida, formulated national cancer screening guidelines in 2013. Based on this, VIA was considered as an effective screening strategy in countries like India, where resources for cytology are scarce. Subsequently, it was included in the NPCDCS program and implemented in all the states to the last mile involving grassroots workers like village health nurses and Accredited Social Heath Activist (ASHAs).
In 2018, the Federation of Obstetric & Gynaecological Societies of India (FOGSI) developed resource-stratified Good Clinical Practice Recommendations (GCPR) or screening and management of screen-positive cases by stratifying the healthcare system into good-resource settings and low-resource settings. This helps clinicians to choose the appropriate method of screening based on available resources and individual preferences.57
In 2019, the National Cancer Grid, a consortium of more than 180 cancer institutions in India, which aims to provide evidence-based guidelines on the three most common cancers in India produced population-based screening strategies for breast, cervix, and oral cavity cancers. VIA is considered as a viable option of screening women aged 30–65 years one to three times in their lifetime.58
The Department of Health Research has released a health technology assessment for early diagnosis of cervical cancer. Based on this, screening is the major cornerstone in the prevention paradigm, and it suggests VIA every 5 years as the most cost-effective screening method in the context of India presently.59
DEFICIENCIES TO BE ADDRESSED
Gaps in the implementation of screening and awareness activities
Various barriers, including the health system, provider and community-related socioeconomic and cultural issues, have slowed down the screening efforts in developing countries [Figure 7].
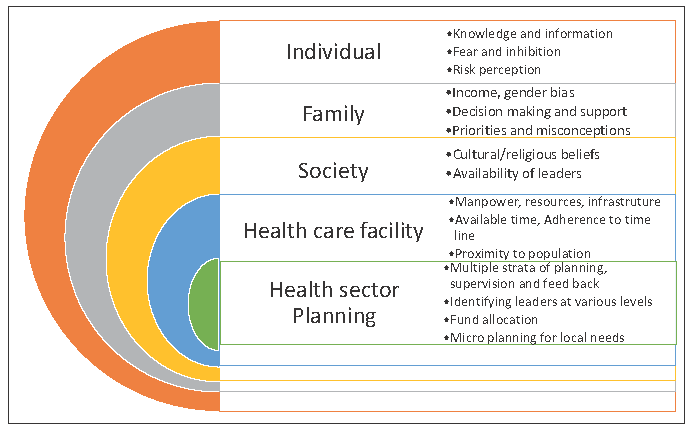
- Barriers in cervical cancer screening.
Health system
The national guidelines for screening of common cancers, including cervical cancer, were introduced in 2016, but they still face several lacunae in terms of implementation. India introduced and scaled up VIA-based programs with varying levels of organization and performance. Case studies have shown that program organization rather than a choice of test may determine the success of a screening program.60 Due to the simplicity of VIA, it was possible to build infrastructure, increase numbers of trained health care personnel, and develop a system of multilevel coordination within the health system. However, after more than 7 years, the number of women screened remains very low (∼3%). A major reason for this could be a lack of political will and the absence of a dedicated advisory body for prevention activities at various levels. Shifting of priorities in health care with a focus on the increasing burden of cancer is required. The major lacunae faced are fund allocation, human resource building, infrastructure, and equipment. Furthermore, communities have poor access to the health care system, which increases the gap even further. Implementation of simple screening algorithms and ensuring an affordable and continuous supply of high-performance screening test will help achieve the goal.
Provider
Rigorous training and retraining of health care workers at primary and secondary levels for performing VIA and maintaining quality control, as well as timely referral and treatment of positive cases is still lacking. There are no fixed protocols or a dedicated facility for cervical cancer screening at health care centers. Continuous supply of affordable screening test kits is a challenge faced at many places. The screening test chosen, i.e. VIA, lacks high sensitivity and is dependent on human resources and, therefore is not able to meet community needs. HPV DNA testing kits, along with point of care (POC) tests have performed far better, but their availability is a major issue at present. Women who are screened positive face difficulties in reaching referral centers or getting treatment, and this leads to high losses to follow-up. There is no database or health information system to track these women. The option of self-sampling is still on a research basis, although it has the potential to reduce the burden on health care workers and mobilize women who are not willing for a pelvic exam. Regular outreach camps in difficult terrains are still lacking.
Community
The major problem highlighted in several studies remains a lack of awareness regarding this preventable cancer among the population who are at risk. There are several sociocultural aspects leading to women not prioritizing screening. One of the reasons could be that cancer is not considered a curable entity. Information, education, and communication regarding the causes, and primary and secondary prevention through audiovisuals in the native language could help in reaching to the masses, but they are nonexistent at present. The social structure of a community generates a lot of myths and misconceptions regarding mass screening in camps, leading to poor turnover. Fear and inhibition, along with loss of daily wages incurred, gender bias, and cultural beliefs, add to the problem.
HPV infection, being a sexually transmitted disease, often leads to the general public questioning the screening due to social reasons. The inclusion of ground-level healthcare workers in dispelling myths and addressing the needs of the community with an understanding of its social structure is a necessity.
Figure 8 depicts the major gaps to be addressed at these three levels.
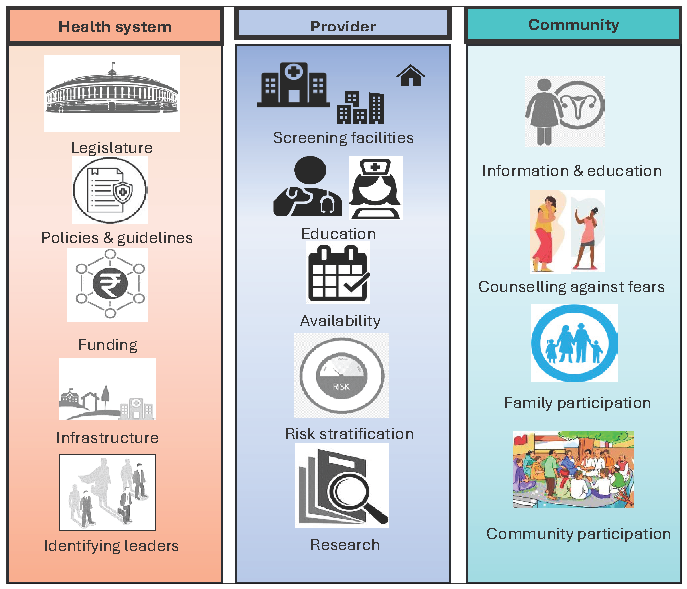
- Major gaps to be addressed at the level of health system, provider, and community.
Mitigating the gaps
While implementing a nationwide policy, the program should address the needs of the local population. The attitude of women who undergo screening defines the direction of the rest of the people in their locality. The feedback from the women who are undergoing screening, to a great extent, influences the others. Hence, educating and screening every woman who enrolls for screening, keeping her confidence intact, and promoting her as an ambassador for the cause will amplify the outcome. Training and including the local women to be part of the team for screening will promote screening by increasing familiarity among the local population and also will help to get feedback.
The planning should be done separately for program managers, healthcare workers, and the targeted population. Time and resources invested in pre-implementation field work and microplanning will help to increase the percentage of women screened. Legislature directing employers to screen women undergoing medical examination during induction into a job or undergoing medical review is the need of the hour.
Follow-up of women undergoing screening and informing them of their results adhering to the timelines will motivate them. The screening team should also convey the management plan for screening-positive women when the reports are conveyed. The delays in management after conveying the positive screen test report will bring more apprehension and attrition. A facility to triage or treat should be available near the screening setup. Reducing the number of visits from screening to treatment is mandatory.
We need implementation studies to understand the impact of screening and the factors that alter the uptake and outcomes of screening. The implementation studies will help to pick up the positive aspects, drop the negative ones, and change the strategy when required.
Population-based data on screening, i.e., the number of women who have undergone screening, the method used, and their follow-up cum referrals, are not available. Creating a nationwide registry for screening linked with a permanent identification number like an Aadhar number may help to understand the ongoing activities, planning, and implementation.
Innovations in the areas of newer screening methods, triage tests to deliver treatment at the same visit, identifying risk factors for the increase in adenocarcinomas of cervix, identifying risk factors in new generations with changing family patterns, development of portable colposcopy, incorporation of artificial intelligence into screening, self-screening methods, using molecular markers for screening, innovating new ways for understanding the KAP, new methods for information-education and counseling (IEC) activities, designing and testing a population-based screening registry, and training for undergraduate medical students in screening activities are still awaited. The existing screening algorithms need field implementation trials in a large population cohort.
Allocation of funds for cancer prevention research will help to increase the number of research activities in this area of study.
Transition to screening by HPV DNA testing (including POC tests and self-sampling)
Despite the plethora of screening tests [high-risk HPVDNA/Nucleic Acid Amplification Test (NAAT)/mRNA tests, VIA, Liquid-Based Cytology, and Pap Test] being available, the acceptance of these tests is largely influenced by accessibility to health care facilities, socioeconomic status, and awareness. Only tests validated by international standards that have clinical sensitivity rather than analytical sensitivity should be used.
HPV DNA testing has higher sensitivity (96.1% vs. 53.0%) but lower specificity (90.7% versus 96.3%) when compared to cytology. several multicentric trials have established that primary HPV testing provided about 60–70% greater protection against invasive cervical carcinomas as compared to cytology.61
WHO recommends using HPV DNA detection as the primary screening test rather than VIA or cytology in screen-and-treat/screen-triage and treat approaches among both the general population of women and those living with HIV (strong recommendation with moderate certainty evidence).30 HPV mRNA tests are also available, but these are not recommended for use in self-sampling.
Self-sampling
HPV testing can be done on provider- or self-collected samples. Although many commercially available hrHPV kits (cobas®, BD OnclarityTM, Aptima®) are available for screening, only COBAS 4800, 6800, 8800, and BD Onclarity are Food and Drug Administration (FDA) approved for primary cervical cancer screening. Unfortunately, in many countries, especially LMICs, standard cervical cancer screening tests are not universally or even widely available, and hrHPV primary screening is limited due to cost and logistics issues. Women may feel shame and embarrassment due to personal or sociocultural reasons, and in such circumstances, self-sampling can circumvent the hesitancy in treatment-seeking behavior. Self-sampling can help in reaching women residing at the last mile.
A meta-analysis of 18,516 female participants from 24 countries across 5 continents showed that 65% women preferred self-sampling over physician sampling; the reasons quoted were ease of use, not embarrassing, privacy, comfort performing self-sampling, ability to sample on their own, and convenience.62 In another study, authors noted that hrHPV assays based on PCR were equally sensitive on self-samples as well as clinician samples to detect preinvasive lesions (CIN2+ or CIN3+). Also, self-sampling by mailing the kits to women’s home address generated a much higher response rate as compared to physician sampling. There was a 12% reduction in sensitivity for the detection of CIN2+ when self-sampling was compared with clinician-collected samples, but this was only seen when testing was performed using hybridization signal-based assays (example: Digene HC2 assay). It is interesting to note that this reduction in sensitivity was not seen when HPV testing was performed using amplification-based methods such as PCR.63
In a study among rural women in India evaluating the acceptability of self-sampling on a five-point Likert scale on parameters like ease, privacy, and discomfort, it was observed that self-sampling was significantly preferred over physician sampling. They used the HC2 test to process both sampling techniques, and it was found that there was a substantial level of concordance between the two methods (Cohen’s kappa – 0.73, 95% CI: 0.34, 1.00).64
Point of Care (POC) testing
POC testing overcomes limitations like cost, processing time, and laboratory infrastructure and facilitates the screen-and-treat approach in a single visit. They are highly efficacious in LMICs and high-risk HIV populations where women with positive HPV DNA test can be counseled and offered further evaluation to assess their eligibility for thermal ablation/cryotherapy on the same day. Widely used and commercially available tests include:
-
1.
CareHPV (Qiagen): Based on chemiluminescence technology, this qualitative test provides test results within 3 hours.
-
2.
Xpert HPV (Cepheid Diagnostics): This includes DNA extraction, amplification, and detection using PCR technology in integrated cartridges and provides reports as HPV16, HPV18/45, or other hrHPV (31, 33, 35, 52, 58; 51, 59; 39, 56, 66, 68) positive within 60 minutes.
-
3.
Truenat (Molbio Diagnostics, Goa, India): This detects four hrHPV types: 16/31,18/45 via a microchip-based real-time PCR assay; test results are available in 60 minutes. Now a modified version with 8 types is undergoing international validation testing. It has HPV 16/18 in one channel and HPV 31, 33, 35, 45, 52, 58 in the other.
Partial genotyping in these tests provides an in-built triage method. Other POC tests are currently under development. Various studies have validated the use of POC tests on self- and physician-collected samples in community settings. A study conducted in South Africa evaluated the diagnostic accuracy of Xpert HPV (five channel reporting – HPV type 16; HPV types 31, 33, 35, 52, or 58, or more than one of these types; HPV types 18 or 45, or both; HPV types 51 or 59, or both; and HPV types 39, 56, 66, or 68, or more than one of these types) in detecting CIN 2+ and higher lesions in HIV-positive and HIV-negative populations. Sensitivity in HIV-negative women for all channels was 88.7% (95% CI 83.1–94.3) while specificity was 86.9% (95% CI 83.4–90.4). In HIV-positive women, sensitivity was higher 93.6% (95% CI 90.0–97.3), but specificity was lower 59.9% (95% CI 54.1–65.7) as compared to the general population.65
An Indian study evaluated the diagnostic value of a POC, test Truenat, which detects four hrHPV genotypes (16, 18, 31, and 45), using HC2 as a reference test. Of 615 cervical samples, 78 (12.7%) women were found to be hrHPV DNA positive by HC2 and 49 (8%) by Truenat. Keeping in mind the limited genotype testing offered by Truenat, its sensitivity and specificity were found to be 97.7% and 98.9%, respectively.66
Is POC testing really the way forward in LMICs where the screen-and-treat/single-visit approach is technically more feasible than the two-visit approach? To evaluate this, a modeling study evaluated the monetary benefit from a single-visit approach as compared to a two-visit approach in three LMICs (India, Nicaragua, and Uganda) using a mathematical simulation model of the natural history of HPV and cervical cancer. Outcomes included health benefits measured as a reduction in lifetime risk for cervical cancer incidence and lifetime costs. Screening at least three times in a lifetime at 30, 35, and 40 years with a two-visit vs. one-visit strategy at a lost to follow-up (LTFU) rate of 10% had a similar reduction in rates of cervical cancer: 62.0% vs. 65% in India, 66.0% vs. 68.8% in Nicaragua, and 67.4% vs 70.1% in Uganda. But as LTFU increased with the one-visit strategy, the reduction in cancer risk remained stable in each country, while with the two-visit approach, it diminished substantially. Also, as LTFU increased, reducing the number of clinic visits (shifting from two-visit to one-visit strategy) was found to be cost-effective.67
In the last two decades, there have been consistent efforts to develop low-cost indigenous POC devices. In the field settings of primary health centers, the only available light source is generally a tungsten bulb emitting yellow light attached to a torch or examination light. An ideal light source with certain magnification was a highly desirable requirement for visual inspection under magnification of the cervix. A portable, user-friendly, low-cost device (US$160 per piece, AV Magnivisualizer), which has a complete spectrum of visible light (white light) and interchangeable magnification, has been launched by the Government of India for widespread use. A study to evaluate the device showed better sensitivity to detect precancerous lesions of the cervix compared with VIA (83% vs 54%) without loss of specificity. The authors concluded that the AV Magnivisualizer may be useful in settings where colposcopy facilities do not exist.68
Improvement of referral system; training primary and secondary health care workers
The referral system from the place of screening to the place of management plays a vital role in every aspect. The staffing needed to manage screen-positive women appropriately necessitates decentralizing and bringing management interventions to or near the screening sites. Multiple referrals lead to attrition in the number of patients availing of the proper treatment.
India’s primary health care system focusing on reproductive and child health activities are in four tiers: subcenters (SC), primary health centers (PHC), community health centers (CHC), and district/sub divisional hospitals. Community health centers implement national health programs which are involved in cancer prevention. The operational framework for the prevention of cancer guides all four tiers to participate in cancer prevention activities.
Observations from the District Level Household and Facility Survey (DLHS-4) suggested that there was significant heterogenicity in facility readiness for cancer screening in all four tiers of the healthcare system. Infrastructure and staffing were the substantial barriers to screening.69 Experience from the past clearly shows that implementation strategies for cervical cancer screening should be at multiple levels, including a diverse set of stakeholders planning screening and treatment. Task-sharing strategy allocating responsibilities to peripheral health setups needs vigorous preimplementation activities, which will increase the knowledge, motivation, and leadership among peripheral workers. Figure 9 shows the key components of improving the referral system.
- Components of improving the referral system.
Manpower
While many states have succeeded in initiating task shifting and have a pool of trained manpower, the practice of frequent transfers results in loss of expertise in many instances. Increasing the manpower at peripheral setups with a clearly defined job description is ideal, to keep a high quality of screening and management activities. Manpower calculations should be dynamic with provision to increase the number based on future escalation of the program. The provision of patient coordinators who can facilitate the referral pathway and help clients to access services at the referral centers will improve participation rates.
Training
Ongoing training and refresher training of manpower is essential for quality assurance. Training centers at district levels with fixed training calendars and participation of already trained staff in ongoing training activities will increase the confidence to deliver the expected services. Structuring a training module for every level of care involved in cervical cancer screening and management with specified goals and referral guidelines will help to standardize the quality of care. Staff also needs to be trained to handle digital platforms. Recruitment of manpower or agencies to manage social media and other mediums of communication and data collection, which will improve IEC activities and capture data on KAP, will provide future directions to the program.
Data management and tracking
Ensuring smooth communication channels between primary and secondary care facilities will allow for timely patient information and records transfer. Developing software programs enabling monitoring and follow-up of medical records from the peripheral centers will improve the quality of service. Electronic medical records will help identify the gaps and delays in the referral system.
Innovations
Testing and including newer treatment methods like thermal ablation and other contemporary screening and treatment strategies at peripheral setups will help to facilitate the services provided. Including innovations like portable colposcopes at screening setups will help to strengthen the referral system by avoiding unnecessary referrals/undertreatment. Including telemedicine, teleconsultation, and tele-mentoring facilities will reduce the number of visits for women undergoing screening. Ongoing learning programs through telemedicine will gradually increase the capacity of peripheral setups and help in task-sharing. Incorporating artificial intelligence (AI) for diagnosing and AI algorithms for deciding on management will reduce referrals.
Incentives and motivation
The performance of the referral system at every level should be encouraged by recognizing their work and motivating them with incentives. Monetary rewards, awards, and other incentives for excellence in work can improve performance.
Community participation
The role and contributions of ASHA workers in providing health care in the periphery are significant. Including ASHA workers, community activists, local leaders, and NGOs as part of the referral system and utilizing their services where and when required will bring better outcomes.
Continuous quality improvement
Monitoring the ongoing screening, referral, and training activities through various channels is mandatory for quality improvement. Regularly collecting feedback from the target population and healthcare workers will improve the referral system.
Introduction of HPV vaccine in the national program
The inclusion of an affordable prophylactic HPV vaccine into the national immunization program can significantly alleviate India’s cervical cancer burden. Addressing vaccination-related myths and stigma through mass awareness campaigns is vital to boost acceptance rates. HPV vaccination offers safe and effective protection against HPV infections that lead to cancer, marking a crucial step toward eliminating cervical cancer.
Global experiences with HPV vaccination
Several countries have already integrated HPV vaccines into their national immunization programs, leading to a decline in the prevalence of high-risk HPV infections and earlystage cervical cancer cases. By examining these global experiences, India can learn from both successes and challenges encountered during vaccine implementation. Australia, Canada, the United Kingdom, several European countries, Rwanda, Malaysia, and Bhutan have integrated HPV vaccination into their immunization programs, leading to a decline in HPV infections and related cancers. Australia’s school-based HPV vaccination program, initiated in 2007, has demonstrated a remarkable reduction in HPV prevalence, signaling the vaccine’s effectiveness. A decline in cancers has been reported from Nordic registries and from the UK. Similarly, Rwanda’s proactive approach to nationwide vaccination campaigns has shown the potential for high coverage rates, which is essential for achieving population-level protection.
Measures for a successful introduction in the national immunization program
-
1.
Policy and Advocacy: Efforts must be made to advocate for the inclusion of HPV vaccines in the national immunization program. Policymakers, healthcare professionals, and advocacy groups need to collaborate to build a robust policy framework for vaccine integration.
-
2.
Vaccine Accessibility and Affordability: Ensuring that HPV vaccines are accessible and affordable to all eligible population is crucial.
-
3.
Health Care Infrastructure and Training: The successful implementation of HPV vaccination requires a well-established health care infrastructure and adequately trained health care personnel. Strengthening health care facilities and providing training for health care professionals are essential steps in this direction.
-
4.
Addressing Vaccine Hesitancy: Vaccine hesitancy remains a challenge in many regions. To gain public trust, it is essential to address concerns related to vaccine safety, efficacy, and potential side effects through targeted communication and education campaigns.
-
5.
School-Based Vaccination Programs: Incorporating HPV vaccination in school-based programs can reach a large number of eligible girls and ensure widespread coverage.
-
6.
HPV Vaccination and Equity: It is crucial to address equity concerns to ensure that HPV vaccination reaches all eligible populations regardless of SES, geographic location, or cultural beliefs. Special attention should be given to marginalized communities to prevent further disparities in cervical cancer prevention.
-
7.
Monitoring and Surveillance: A robust monitoring and surveillance system is essential to evaluate the impact of HPV vaccination on cervical cancer incidence and to detect any potential adverse events.
-
8.
Communication and Awareness: Effective communication and awareness campaigns are vital to inform the public, health care providers, and policymakers about the benefits of HPV vaccination and cervical cancer prevention. Tailored messages and culturally sensitive approaches can help maximize vaccine uptake.
In conclusion, the introduction of HPV vaccination in India’s national immunization program holds tremendous potential in reducing the burden of cervical cancer. However, several deficiencies need to be addressed to ensure successful integration and equitable access to the vaccine. By learning from global experiences and developing evidence-based strategies, India can take significant strides in combating cervical cancer and improving women’s health nationwide.
Improving access to radiation and chemotherapy
India’s oncology community acknowledges acute shortages of vital resources even for fundamental cancer management. The scarcity of radiation and chemotherapy resources disproportionately affects vulnerable populations, particularly rural areas. These therapies are not only crucial for LACC management but also offer palliative care for those with incurable disease. The key barriers are:
Geographical disparities: The concentration of treatment centers in urban areas creates an imbalance in access, leading to delayed or inadequate care for those living far from medical facilities.
Infrastructure deficits: The shortage of equipped radiotherapy and chemotherapy centers further exacerbates the problem. Insufficient facilities and outdated equipment hinder the delivery of timely and effective treatment, thereby affecting patient outcomes.
Financial constraints: Cervical cancer treatment can be financially burdensome, and many patients face difficulties in affording the costs associated with radiotherapy and chemotherapy. High out-of-pocket expenses, coupled with limited health insurance coverage, deter patients from seeking appropriate care.
Lack of awareness: A lack of awareness about cervical cancer and its treatment options among the general population and health care providers leads to delayed diagnosis and treatment initiation. This contributes to the advanced stage at which many patients are diagnosed, making treatment less effective.
Health care workforce shortage: There is a shortage of trained oncology health care professionals, including radiation oncologists and medical oncologists, which impacts the capacity to deliver radiotherapy and chemotherapy services to the growing number of cervical cancer patients.
Allocating resources to establish comprehensive treatment facilities in underserved regions can bridge the existing gap in cervical cancer management. The potential strategies for addressing these barriers include:
Policy reforms: Government policies can be used to increase investment in radiotherapy and chemotherapy infrastructure, expand access to affordable cancer care, and raise awareness about cervical cancer.
Infrastructure development: New radiotherapy and chemotherapy centers can be established in rural areas, and existing facilities can be upgraded with modern equipment.
Health care workforce training: Scholarships, training programs, and incentives can be offered to attract more students to study oncology and to encourage qualified professionals to work in rural areas.
Public awareness campaigns: Public awareness campaigns can be used to educate the general population and health care providers about cervical cancer and its treatment options.
Apart from the mentioned challenges and strategies, the lack of comprehensive data on cervical cancer cases and treatment outcomes hampers our understanding of care deficits and effective interventions. Robust data collection and research initiatives are vital to guide evidence-based strategies for better access to radiotherapy and chemotherapy for cervical cancer patients in India. Collaborative efforts between health care institutions, government bodies, and research organizations can strengthen a national cancer registry, enhancing our understanding of the disease landscape. Investing in cervical cancer research is essential for identifying innovative and culturally appropriate diagnostic and treatment approaches tailored to suit resource-limited countries like India.
Research can also help in identifying cost-effective interventions without compromising quality, reducing the financial burden on patients and health care systems. Research can inform strategies to improve treatment delivery, minimize interruptions, and manage side effects, enhancing patient experiences. It is also the need of the hour to develop nation-specific guidelines for cervical cancer treatment. These guidelines should be based on a comprehensive understanding of disease prevalence and treatment responses within the Indian context, ensuring consistent and high-quality care. Evaluating the effectiveness of telemedicine and mobile health units is also essential to improve care in underprivileged areas. Insights can expand these initiatives to reach underserved populations.
Palliative care
Cervical cancer is associated with severe morbidities, painful course, and difficult deaths. It is the most common cause of death from cancer amongst economically poor women. These women suffer from a high prevalence of malodorous discharge, excessive bleeding, severe abdominal pain, sexual dysfunction, and urinary fistulas in advanced stages. These disabling physical symptoms have a significant impact on the psychosocial and emotional well-being of these women. Since the disease is more prevalent amongst the poor socioeconomic strata, cost-effective interventions are necessary.
Appropriate management using oral metronidazole, oral morphine, antidepressants, and laxatives can significantly improve the quality of life of these women. Less than 1% of India’s population has access to palliative care.70 Opioid availability for pain management is scarce, with poor availability of trained staff for prescribing and titrating the medication. The excruciating pain significantly affects the life of both the patient and the caregivers. Palliative care specialists can adequately and safely provide pain management to these women. The availability of these pain-relieving medications, along with appropriate training of the specialists for pertinent management is an essential component of palliative care in these women.
Women with advanced cervical cancer usually visit the emergency room in uremia secondary to ureteral obstruction due to various causes. Decompression of obstructed ureters using ureteral stunts or percutaneous nephrostomy (PCN) is a management option. Though seen as an emergency “life-saving option” by the physician and the patient, the decision should be based on clear communication about the prognosis, benefits, and burdens of this intervention.71
Palliative radiotherapy is a cost-effective intervention to reduce vaginal discharge, bleeding, pressure effects, and nociceptive pain caused by pelvic and para-aortic disease. Simple and safe regimens, though are still not conceptualized well.
Palliative care is essential for women with advanced cervical cancer, but it is usually administered when curative treatment is no longer feasible. There is growing evidence that early integration of palliative care (EIPC) with ongoing oncological management can significantly improve the quality of life (QoL) for these women.72
In conclusion, the suffering of women with advanced cervical cancer, an illness mainly of the poor, has been ignored by scientific research with no established mechanisms and protocols for providing palliative care to these women. The collaborative effort of researchers, public health officials, oncologists, gynecologists, and primary care providers should be the basis of palliative care in these women.
RECOMMENDATIONS FOR WAY FORWARD
Implementation of WHO’s cervical cancer elimination initiative
In 2018, the Director General of WHO, Dr. Tedros Adhanom Ghebreyesus, issued a call for action to scale up prevention, detection, and treatment to finally eliminate cervical cancer as a public health problem. In 2020, WHO’s member states responded to the call, passing a historic resolution with specific target goals through the World Health Assembly. On November 17, 2020, WHO launched the three-pillar strategy. The resolution and the strategy established clear targets to achieve by 2030:
-
1.
HPV vaccination coverage – 90% of girls are fully vaccinated by HPV vaccine by 15 years of age.
-
2.
Screening – 70% of women are screened by a high-performance test by the age of 35 years and again by the age of 45 years.
-
3.
Access to treatment – 90% of women with pre-cancer were treated and 90% of women with invasive cancer were managed.2
Now, implementing the cervical cancer elimination initiative involves a multifaceted approach encompassing vaccination, screening, treatment, awareness, and collaboration among stakeholders.
HPV vaccination
Central to the initiative is the widespread availability and administration of HPV vaccine. The initiative requires strong partnerships with governments, international organizations, and pharmaceutical companies to ensure affordable and equitable access to these vaccines, particularly in low-income countries. Educational campaigns are essential to dispel myths and ensure public acceptance of vaccines. Based on the WHO data available, by the end of 2022, the number of manufacturers with licensed HPV vaccines and production capacity has also increased rapidly. India developed its own indigenous quadrivalent HPV vaccine (Cervavac, SIIPL) in September 2022. The Indian government is planning to roll it out in the government sector in three phases for 9- to 14-year-old girls in the near future. With this three-phase strategy, 68 million girls in India would have been vaccinated, and a further 11.2 million girls aged 9 years will be targeted for routine HPV vaccination year.73
Based on the latest evidence, WHO/SAGE issued recommendations on the possibility for adolescents up to age 20 years to receive a single dose, which can reduce cost and increase flexibility to reach higher coverage.20 Since the Call to Action, 141 countries have introduced the HPV vaccine into their national schedule, and 59 countries have a single-dose policy.
Screening
It is of utmost importance to develop organized screening programs for systematic screening, treatment, and follow-up of screen-detected women. Cervical cancer screening programs using cost-effective tests and simple algorithms should be implemented for the early detection of precancerous conditions and cancers. These programs should prioritize accessibility, especially in rural and underserved areas. To enhance screening efficiency, innovative approaches like self-sampling kits can empower women to take control of their health. Integration of screening into existing health care services can maximize the initiative’s reach. Due to the lack of manpower, infrastructure, quality control, and financial resources, high-quality cytology and HPV screening may not be feasible for wide-scale implementation of the cervical cancer screening program in LMICs. Visual screening tests, i.e., VIA/VILI should be adopted till a low-cost/POC reliable HPV test becomes available.25 VIA can be performed by trained doctors and paramedical staff, with adequate training and quality assurance. Adequate knowledge and training of the health care workers is essential before the implementation of the screening programs. Medical camps with VIA as a screening tool can be conducted in rural/slum areas. Looking to the future, WHO is supporting innovation for the next generation of POC testing and artificial intelligence based screening, which holds exciting potential to further improve quality, reduce costs, and increase access.
Treatment
Diagnosed preinvasive and invasive cases must be promptly treated to prevent disease progression. The initiative requires investments in health care infrastructure, ensuring that health care facilities have the necessary equipment and skilled personnel for accurate diagnosis and effective treatment. This may involve training health care professionals, enhancing laboratory capabilities, and ensuring the availability of treatments like cryotherapy and thermal ablative methods. With recommendations on the use of portable, battery-powered thermal ablation devices to treat precancerous lesions, WHO has also been supporting countries to phase out cryotherapy. In doing so, WHO supported reduced access pricing for thermal ablation products. Collaboration with medical institutions and professional bodies is essential to ensure standardized treatment protocols. For the management of invasive cancers, WHO has the capacity to strengthen and scale up services and provide guidance for procuring radiotherapy devices. WHO deployed clinical experts to help train surgeons and oncologists, established new partnerships on diagnostics and radiotherapy, and brought together professional societies to further build capacity in LMICs.
Awareness and education
Public health campaigns should target communities, schools, workplaces, and media outlets to disseminate accurate information and dispel misconceptions. The involvement of local leaders, women’s organizations, and community health workers can facilitate culturally sensitive conversations. Sex education that addresses HPV transmission and cervical cancer prevention should be integrated into the school curriculum. Sikkim, a state in northeast India that has successfully rolled out HPV vaccination, reached 97% of the target population in its initial campaign. This success is because of good education about the benefits of the HPV vaccine and good communication with the teachers, parents, and health care workers before the vaccine rolled out. This approach holds the same with the other two pillars of WHO strategy as well.
Collaboration among stakeholders
The successful implementation of the initiative relies on collaboration among various stakeholders, including governments, international organizations, health care providers, NGOs, pharmaceutical companies, and communities. Governments play a pivotal role in policy formulation, funding allocation, and program implementation. El Salvador is the first country in Central America to reach WHO’s elimination target in all three pillars and is a very good example of the importance of collaboration with the government and stakeholders in the elimination initiative. The country could attain its elimination target through the successful collaboration of the Ministry of Health in including HPV vaccination in their national immunization schedule, the costs of the vaccine and HPV kits were included in the national budget, and a screen-and-treat approach was implemented in all the screening facilities, thereby reducing the lost to follow-up population. International organizations provide partnerships in technical expertise, funding, and support in resource-limited settings. For example, Gavi, the Vaccine Alliance, is providing support for LMICs to introduce and scale up HPV vaccines, while the Bill & Melinda Gates Foundation supported critical HPV vaccine research. International Atomic Energy Agency (IAEA), IARC, and WHO together assist through joint reviews and strengthening the clinical skills of surgeons/oncologists across LMICs. Collectively, these efforts represent a meaningful starting point to advance this ambitious agenda. Pharmaceutical companies contribute by making vaccines and treatments accessible and affordable.
Data collection and analysis
A robust monitoring and evaluation system is required for tracking progress and identifying areas needing improvement. Data on vaccination coverage, screening rates, treatment outcomes, and disease incidence should be collected regularly and analyzed. This enables evidence-based decision-making, early intervention in underperforming areas, and the identification of trends and challenges. Transparent reporting mechanisms build trust and accountability among stakeholders.
Challenges and mitigation
Implementing the Cervical Cancer Elimination Initiative is not without challenges. These may include financial constraints, limited health care infrastructure, cultural barriers, vaccine hesitancy, and reaching marginalized populations. To mitigate these challenges, a combination of strategies is necessary, including securing sustainable funding through domestic and international sources, strengthening health care systems, conducting culturally sensitive awareness campaigns, investing in research to address vaccine hesitancy, and utilizing mobile technology to reach remote communities.
Screen-and-treat approach for hard-to-reach populations
The uptake of community screening for cervical cancer with existing programs, such as VIA, has several drawbacks. Though a simple procedure, training and, more importantly retaining the trained personnel is a huge challenge. To reach the masses, health care workers in India, such as the ASHAs or community health workers have been successfully trained in the past to tackle health issues at the community level. With one ASHA for approximately 1,000 people, there are now nearly one million ASHAs in the country; they can be a valuable workforce for mobilizing eligible women from the community, counseling them, delivering screening test reports, and recalling screen-positive women for triage and treatment. They have been found to be productive as a result of their proximity to the community, knowledge of the local population, and acceptance by families as providers of different health interventions.74 Using this approach, the screening services can be packaged into the already existing health care system and offer testing at the doorstep, thereby overcoming the hurdles of accessibility, cost of travel, time away from home, vocation, and privacy.
With the introduction of testing for HPV as a primary screening test, self-sampling has gained broad attention due to its potential to increase screening coverage. In camp and outreach settings too, self-collection for HPV testing with a POC test is a good option.
Various other methods to include the hard-to-reach populations have been tried such as indigenous field worker sampling, where the field worker has special training pertinent to the objectives of the study, including interview skills and fieldwork protocols and have privileged access to the target population.75 Another method would be facility-based sampling, which refers to recruiting members of the target population from a variety of facilities, including correctional and drug treatment centers, sexually transmitted disease clinics, or general health centers and hospitals in certain suburban areas. Some members of hidden populations, e.g., migrant workers, tend to gather at certain locations within the community, and therefore time–location sampling is used to recruit these groups of hard-to-reach populations at locations where they may be found at a given time.
In the screen-and-treat approach, the decision to treat is based on a positive primary screening test only without triage (i.e. no second screening test and no histopathological diagnosis). The emphasis is on reducing the burden of an additional visit, thus enhancing the impact of the screening. Depending on the findings of the screening test, if the patient is eligible for ablative treatment, this should ideally be done immediately (the single-visit approach). At some facilities, this is not feasible and a second visit may be needed (the multiple-visit approach). Women who are not eligible for ablation can have excisional treatment on the same day if the clinic has the capacity for large-loop excision of the transformation zone (LLETZ). If LLETZ is not available on-site, women need to be referred for excisional treatment or for further evaluation.
Although the current recommendation is for HPV testing as screening for the screen-and-treat approach, it is also suggested that existing programs with quality-assured cytology as the primary screening test should be continued until HPV DNA testing is operational with respect to cost and quality assurance. Existing programs using VIA as the primary screening test need to be transitioned rapidly to HPV screening, given the inherent challenges with quality assurance and sustenance.
In the screen-and-treat strategy using primary HPV testing, women who are HPV-negative are not treated nor evaluated further. Women who are HPV-positive should all be treated based on the eligibility for ablative treatment with the application of acetic acid and visual evaluation using the naked eye or with a colposcope. Those who are ineligible for ablative treatment should be referred for excisional treatment or further evaluation.
The treatment aims to destroy or remove the entire transformation zone of the cervix, including areas of the cervix that have been identified as abnormal by screening. In the HPV screen–positive women, it has been suggested that they undergo ablation even when there are no acetowhite lesions on acetic acid application. The rationale behind this is that these women are at a high risk of developing a high-grade lesion in the foreseeable future.
Introducing screening in ART centers for WLHIV
WLHIV are up to seven times more likely to develop cervical cancer than uninfected women, the reasons being a higher risk of coinfection with high-risk HPV types, HPV reactivation, and persistence and low regression of HPV infection. Cervical cancer is the most prevalent acquired immune deficiency syndrome (AIDS) defining malignancy.76
Primary HPV screening is considered the standard for cervical cancer screening currently. In the study by Boddu et al.77 (2021), HPV testing had the highest sensitivity (90.9% vs 75%) amongst various screening methods for the detection of high-grade preinvasive lesions with low specificity (68% vs. 83.9%) and diagnostic accuracy (69.4% vs. 83.3%) compared to Pap smear. This can be explained by the high prevalence of HPV infection in HIV-infected women, along with poor clearance, increasing the risk of malignancy.
The effect of antiretroviral therapy (ART) on the incidence of cervical cytological abnormalities remains unclear. Early ART initiation may reduce the risk of coinfection with hrHPV but does not prevent the persistence of the infection. With an increase in overall life expectancy, the risk of development of cervical cancer increases with significantly high morbidity and mortality.
According to WHO 2018 data, there are 18.2 million WLHIV, including 0.9 million women from India. The major challenges that affect the process of cervical screening in India in ART clinics are:
Fatalistic attitude toward the diagnosis of HIV/AIDS.
Difficulty in going to a separate clinic for screening.
Lack of knowledge and social support.76
The prevalence of high-grade neoplasia among HIV-positive women is higher, which is 6.4% as compared to 0.5% in HIV-negative women.77 Prevalence of high-grade neoplasia has been seen to be significantly associated with low CD4 counts in various studies.
The prevalence of HPV infection is high in WLHIV, reported as 37.6%–41%, which is much higher than the general population, i.e., 5.9–6.6%. The data of abnormal Pap smear have been variable ranging from 8.1% to 38.3%, whereas VIA positivity has been noted as 32.2% across various studies78,79.
The evidence assessing the test accuracy of the various screening modalities (Pap smear, hrHPV testing, and VIA) is sparse. A study by Pimple et al. from a tertiary center in India in 2022 provided useful comparable measures of evaluation of three cervical screening tools. It is vital to choose the most feasible and effective screening strategies among WLHIV for implementation in public health programs. The screening tests provided to WLHIV showed high test-positive rates of 35.7%, 34.4%, and 9.6% for VIA, hrHPV, and cytology, respectively, in this study.79
Pimple et al. demonstrated the use of diagnostic colposcopy with or without biopsy in investigating the true prevalence of CIN in HIV-infected women. Pap cytology and diagnostic colposcopy to detect high-grade lesions showed low sensitivity but high specificities with very high positive predictive value.79
WHO recommends screening sexually active WLHIV for HPV or cervical abnormalities as soon as they are diagnosed with HIV, and then rescreening them within 3 years.18 Adding cervical screening to HIV services is costeffective and scalable, yet these integrations are low. The feasible process of integrating cervical cancer screening tools in the STD/HIV/AIDS testing centers, technically known as integrated counseling and testing centers (ICTC), located in government facilities needs to be promoted. However, in most STD clinics, cervical cancer screening is not part of the routine testing offered to women attending the ICTC. Women are encouraged to visit the gynecology OPD after ART clinic appointment. There are several barriers to the process of cervical cancer screening in WLHIV. The stigma related to the increased risk of cancer diagnosis, lack of knowledge, and lack of financial and social support are amongst the many hurdles in the integration on both services.
Changing concepts in surgery
Surgical management of cervical cancer has always been ambivalent with concepts differing in terms of geographical region, histology, surgical expertise, and other factors. The most significant of these is perhaps a change back to the conventional open radical hysterectomy, along with various newer anatomical classifications and concepts of nerve sparing, as per Querleu Morrow. The LACC trial44 showed a poor DFS and OS with a minimally invasive approach, and subsequently, this was incorporated in the International Guidelines wherein the standard and recommended approach for radical hysterectomy is an open abdominal approach.
However, there are ongoing studies in cases with tumor size less than 2 cm, which will give us a concrete answer in the future regarding surgical approach (open vs minimally invasive). Similarly, upcoming data (CONCERV, SHAPE, LESSER trial) suggest a possible role of simple hysterectomy providing similar oncologic outcomes as a radical hysterectomy in selected low-risk tumors [maximum diameter <2 cm, depth of invasion <10 mm, no lymphovasular space invasion (LVSI)].80
Lymph node assessment can be achieved through complete lymphadenectomy or in select institutions with a sentinel lymph node (SLN) algorithm with anintent to decrease the postoperative morbidity of a systematic lymphadenectomy. Trials assessing the safety and oncologic outcomes of SLN algorithm (SENTICOL1 and 2) have established the safety of this technique.81
Since cervical cancer patients who report early enough to fulfil the criteria for being surgical candidates are relatively small in number considering the overall disease burden, it will still take some time to authenticate the surgical guidelines to achieve the optimum DFS and OS.
Newer radiation techniques to mitigate shortages and improve outcomes
RT protocol for cancer cervix
In early stages (IA, 1B1, and 1B2), both RT and surgery have equivalent oncologic outcomes. Decision regarding RT versus surgery is based on several factors, e.g., age, comorbidities, concomitant adnexal pathology, as well as patient preference. Stage 1B3 and beyond are treated by chemoradiation.
The standard treatment protocol is 45–50.4 Gray (Gy) in 25–28 fractions by external beam radiation therapy (EBRT) with weekly cisplatin in eligible patients. EBRT is followed by brachytherapy. Brachytherapy is delivered by intracavitary, interstitial, intravaginal, or hybrid methods. Brachytherapy doses in HDR era is 5–6 Gy in 5 fractions or 7 Gy in 3–4 fractions. At the All India Institute of Medical Sciences, New Delhi, 50.4 Gy in 28 fractions by EBRT followed by 7 Gy in 3 fractions to HR-CTV (high-risk clinical target volume) is the standard treatment regimen (with concurrent chemotherapy).
In a country like India, where there are budgetary constraints, optimal and judicious use of radiation techniques utilizing modern technology is very important to mitigate the lack of resources. Brachytherapy is an essential part of the treatment of carcinoma cervix and uterus. Due to the lack of brachytherapy facilities, there is often a waiting list in most RT centers. On average, waiting time for machine availability is 2–6 weeks. This is even higher (up to 3 months) in high-volume centers where the number of cancer cases requiring radiation treatment is increasing disproportionately in comparison to the availability of radiation equipment. Undue prolongation of overall treatment time compromises the survival outcome of cervical cancer patients treated by RT.82
Advances in RT technique to decrease toxicities
1. Image guided intensity-modulated RT: In a phase III randomized trial conducted in India, late toxicity after image-guided intensity-modulated radiotherapy (IG-IMRT) was compared with three-dimensional conformal radiation therapy (3D-CRT) in women with cervical cancer. IG-IMRT resulted in reduced toxicity with no difference in disease outcomes (grade > 2 late toxicity, 28.1% versus 48.9% (HR 0.50; 95% CI, 0.33 to 0.76; P < .001).83
2. Image-guided brachytherapy: Emerging evidence from prospective studies shows a high rate of local control throughout all stages, superior to two-dimensional brachytherapy, with limited toxicity for each organ site. The EMBRACE – I study utilized magnetic resonance imaging (MRI) and the ongoing EMBRACE – II will also be utilizing functional MRI.84
There is a need to innovate newer techniques/regimes of RT in order to mitigate the lack of resources. Some of the following strategies are suggested to overcome this demand versus supply gap.
Expansion of infrastructure: As per the AERB data, India has about 0.30 RT machines per million population. This is grossly inadequate as WHO has recommended one machine per million population. The health care policy in the future must include galvanizing more RT resources in order to meet the required infrastructure.
Adoption of newer technology in practice: The modern RT facilities like advanced linear accelerator need to be strengthened. With modern advanced linear accelerators, the radiation treatment delivery is faster and thus, more patients can be accommodated during a given period. This will reduce the overall treatment course and the burden on existing infrastructure.
Optimization of the RT resources: Certain patients can be treated on brachytherapy alone rather than external beam RT. Optimal use of the existing brachytherapy machine will offload the EBRT machines which already are sparse in number.
-
Short Hypofractionated RT: (delivering higher dose per fraction)
Hypofractionated course of RT is much shorter in duration and can potentially permit us to treat more patients. This kind of regime has already been in use in certain cancer sites like lung, liver, etc., and may be researched in cervical cancer. This will facilitate the speedy completion of treatment and allow treating of more number of patients at a given time. A recent phase II clinical trial from India involving 41 patients explored this hypothesis. Toxicity was within acceptable limits (one patient with grade 2, and two patients with grade 3 rectal toxicity) and overall outcomes (2-year disease-free survival was 85%, and 2-year OS was 94.5%) were also not compromised.85 Thus, the regularization of such a strategy can benefit a huge number of patients without increasing the burden on doctors and the existing infrastructure.
In summary, effective approaches need to be designed and experimented on existing and upcoming infrastructure. In addition, modern technology needs to be used with prudence so that it benefits a large patient population without increasing the cost.
New vistas in chemo- and immunotherapy in cervical cancer
Treatment of locally advanced disease
The benefit of adding a radio-sensitizing agent, cisplatin, to radiotherapy has been proven in five phase 3 trials. A meta-analysis of 19 randomized controlled trials between 1981 and 2000, including 4580 patients, established an improved OS (HR0.71, p < 0.0001) and PFS (0.61, p < 0.0001), with chemoradiation.86
The OUTBACK trial assessed the addition of adjuvant chemotherapy following chemoradiotherapy to LACC.87 In this phase III multicentric trial, 926 patients with stage IB2-IVA disease were randomized to receive standard cisplatin-based chemoradiotherapy alone or chemoradiotherapy followed by adjuvant chemotherapy with four 3-weekly cycles of carboplatin and paclitaxel every 21 days. There were no differences in OS and PFS between the two arms, although the adjuvant chemotherapy arm experienced more grade 3 or worse adverse effects (81% versus 62%, p < 0.0001). However, 22% of the experimental arm declined adjuvant chemotherapy, likely due to residual adverse effects of the primary chemoradiation. Whether a more tolerable short course of chemotherapy prior to chemoradiation might improve patient outcomes will be answered by the ongoing INTERLACE trial (NCT01566240).
Treatment of metastatic/recurrent disease
-
1.
Doublet chemotherapy: Metastatic or recurrent lesions, which cannot be excised or irradiated, are treated with palliative chemotherapy. Dual agent therapy with a platinum agent and paclitaxel has a higher response rate (36% vs. 19%) and improved PFS (4.8 vs. 2.8 months; P > .001) compared to single-agent cisplatin, although the median OS remained 6–7 months.88
-
2.
Targeted therapy: Bevacizumab, a humanized VEGF-neutralizing monoclonal antibody, targets tumor angiogenesis. GOG 240, a phase III randomized clinical trial, examined the addition of bevacizumab to doublet chemotherapy regimens (cisplatin/paclitaxel or topotecan/paclitaxel) in patients with metastatic, persistent, or recurrent cervical cancer. The final analysis revealed that adding bevacizumab improved mean PFS (8.2 vs. 5.9 months, HR 0.68; 95% CI 0.56–0.84; P=0.0002) and mean OS (16.8 vs. 13.3 months, HR 0.77; 95% CI 0.62–0.95;P=0.0068) compared to chemotherapy alone.55
The FDA approved bevacizumab for treating recurrent, metastatic, or persistent cervical cancer in August 2014, and its combination with paclitaxel and a platinum agent or topotecan forms the current first-line standard of care.
Immunotherapy
In the KEYNOTE-158 trial, a phase II study of pembrolizumab monotherapy in recurrent/metastatic cervical cancer regardless of tumor PDL1 expression, objective response rates were 12.2% in the entire cohort and 14.6% in PD-L1-positive tumors. Accelerated approval of pembrolizumab was granted by the FDA for the treatment of PD-L1 positive advanced cervical cancer with disease progression after first-line chemotherapy.
KEYNOTE-826 trial, a multicenter randomized trial, analyzed the benefit of adding pembrolizumab to paclitaxel and cisplatin/carboplatin (with or without bevacizumab) as first-line therapy. ORR were 68% and 50% with a median duration of response of 18.0 and 10.4 months, respectively. Based on these results, the FDA granted regular approval to pembrolizumab for the first-line treatment of PD-L1-positive cervical cancer on October 13, 2021.56
Conjugated monoclonal antibodies: Tisotumabvedotin is an antibody–drug conjugate directed against tissue factor (TF), a protein prevalent in solid tumors. This ADC binds to TF on target cells and is internalized to release monomethyl auristatin E (MMAE), a microtubule-disrupting agent, arresting the cell cycle arrest and prompting apoptosis. The mechanism of anti-tumor action is multifold, including bystander cytotoxicity and immunogenic cell death. In the GOG-3023/ENGOT-cx6/innovaTV 204 study in patients with recurrent/metastatic cervical cancer who received tisotumab vedotin every 3 weeks, ORR was 24% with 7 complete and 17 partial responses, with a median response duration of 8.3 months (95% CI: 4.2, NR).89 FDA granted accelerated approval to tisotumab vedotin-tftv to treat recurrent or metastatic cervical cancer with disease progression after chemotherapy on September 20, 2021. A combination of tisotumab vedotin with carboplatin, bevacizumab, and pembrolizumab is currently under investigation (NCT03786081).
Recurrent and metastatic cervical cancer was once treated with palliative intent. However, recent introductions of targeted and immunotherapy have produced increasing response rates and duration of treatment responses. Treatment goals should include symptom relief, minimal toxicity, and participation in clinical trials.
OPERATIONAL DEFINITIONS OF THE TERMS USED IN THE REPORT
Age-standardized rate – Summary rate that would have been observed, given the schedule of age-specific rates, in a population with the age composition of some reference population, often called the standard population.
Crude Rate – Number of new cases (or deaths) occurring in a specified population per year, usually expressed as the number of cases per 100,000 population at risk.
Cumulative risk – Combination of risks posed by aggregate exposure to multiple agents or stressors in which aggregate exposure is exposure by all routes and pathways and from all sources of each given agent or stressor.
Screen Positive – Women with a positive result on any screening test (HPV test, cytology, VIA).
High-Risk HPV – HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.
References
- Ministry of Health and Family Welfare: Government of India. Operational Framework – Management of Common Cancers. [accessed 2023 Aug 8]. Available from: http://cancerindia.org.in/wpcontent/uploads/2017/11/Operational_Framework_Management_of_ Common_Cancers.pdf [Accessed August 8, 2023].
- WHO Cervical cancer elimination initiative: From call to action to global movement. Available from: https://apps.who.int/iris/handle/10665/336583. [Last accessed15 Jul 2023].
- The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: A register-based observational study. Lancet.. 2021;398:2084-2092.
- [CrossRef] [PubMed] [Google Scholar]
- HPV Vaccination and the risk of invasive cervical cancer. N Engl J Med.. 2020;383:1340-1348.
- [CrossRef] [PubMed] [Google Scholar]
- ICO/IARC Information Centre centre on HPV and Cancer cancer (HPV Information Centre). Human papillomavirus and related diseases in India. Summary Rreport 10 March 2023. Available from: https://hpvcentre.net/statistics/reports/XWX.pdf [Last accessed 5 September 2023]
- Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A cancer journal for clinicians.. 2024;74:229-63.
- [CrossRef] [PubMed] [Google Scholar]
- National Cancer Registry Programme (Indian Council of Medical Research). Report of national cancer registry programme; 2020. Available from: https://ncdirindia.org/All_Reports/Report_2020/resources/NCRP_2020_2012_16.pdf. [Last accessed 15 Jul 2023]
- Educational status, cancer stage, and survival in south India: A population-based study. JCO Glob Oncol.. 2020;6:1704-1711.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology of cervical cancer with special focus on India. Int J Womens Health.. 2015;7:405-14.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Risk factors for cervical dysplasia in Kerala, India. Bull World Health Organ.. 1999;77:281-3.
- [PubMed] [PubMed Central] [Google Scholar]
- Chlamydia trachomatis infection co-operatively enhances HPV E6-E7 oncogenes mediated tumorigenesis and immunosuppression. Microb Pathog.. 2023;175:105929.
- [CrossRef] [PubMed] [Google Scholar]
- Sexual risk factors for cervical cancer among rural Indian women: a case-control study. Int J Epidemiol.. 1997;26:491-5.
- [CrossRef] [PubMed] [Google Scholar]
- Cervical cancer and hormonal contraceptives: Collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet.. 2007;370:1609-21.
- [CrossRef] [PubMed] [Google Scholar]
- Knowledge, attitude, and practice on cervical cancer and screening among women in India: A review. Cancer Control.. 2021;28:10732748211010799.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Rural cancer registry at Barshi, Maharashtra and its impact on cancer control. Natl Med J India. 2010 Sep-Oct;23:274-7.
- [PubMed] [Google Scholar]
- Human papillomavirus-type distribution in women with and without cervical neoplasia in north India. Int J Gynecol Pathol.. 2008;27:426-30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- HPV Genotypes distribution in Indian women with and without cervical carcinoma: Implication for HPV vaccination programme in Odisha, Eastern India. BMC Infect Dis. 2017;17:30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010-2019. Prev Med.. 2021;144:106399.
- [CrossRef] [PubMed] [Google Scholar]
- Optimal human papillomavirus vaccination strategies to prevent cervical cancer in low-income and middle-income countries in the context of limited resources: A mathematical modelling analysis. Lancet Infect Dis.. 2021;21:1598-1610.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Human papillomavirus vaccines: WHO position paper, ; December 2022. Available from: https://www.who.int/publications-detail-redirect/who-wer9750-645-67 [Last assessed 08 August 2023].
- Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multicentre, prospective, cohort study. Lancet Oncol.. 2021;22:1518-1529.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multicentre prospective cohort study. Lancet Oncol.. 2016;17:67-77.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Are two doses of human papillomavirus vaccine sufficient for girls aged 15-18 years? Results from a cohort study in India. Papillomavirus Res.. 2018;5:163-171.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evaluation of immune response to single dose of quadrivalent HPV vaccine at 10-year post-vaccination. Vaccine.. 2023;41:236-245.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cervical Cancer: Formulation and Implementation of Govt of India Guidelines for Screening and Management. Indian J Gynecol Oncol.. 2022;20:4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evidence-based impact projections of single-dose human papillomavirus vaccination in India: A modelling study. Lancet Oncol.. 2022;23:1419-1429.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer.. 2006;119(5):1095-101.
- [CrossRef] [PubMed] [Google Scholar]
- Situational analysis of cervical cancer screening coverage at tertiary care institutes of India. Asian Pac J Cancer Prev.. 2023;24:4269-75.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Performance of a Cartridge-Based Assay for Detection of Clinically Significant Human Papillomavirus (HPV) Infection: Lessons from VALGENT (Validation of HPV Genotyping Tests) J Clin Microbiol. 2016;54:2337-42.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention (2nd ed). Geneva: World Health Organization; 2021. Available from: http://www.ncbi.nlm.nih.gov/books/NBK572317/ [Last accessed 24 Jul 2023]
- Implementation of population-based cancer screening programme in a pilot study from India: Views from health personnel. Indian J Community Med.. 2019;44:68-70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Establishing Baseline Cervical Cancer Screening Coverage - India, 2015-2016. MMWR Morb Mortal Wkly Rep.. 2019;68:14-9.
- [CrossRef] [PubMed] [Google Scholar]
- Human Papillomavirus and Related Diseases Report. India. HPV Information centre. 2021. Available from: https://hpvcentre.net/statistics/reports/ IND_FS.pdf. [Last accessed 08 August 2023].
- Prevalence of cervical cancer screening and awareness among women in an urban community in South India—a cross- sectional study. Ann Glob Health.. 2020;86:30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- How do Indian states handle cancer screening among women? NFHS-5 data reveals. 2020. Down to Earth. Available from: https://www.downtoearth.org.in/blog/health/how- do-indian-states-handle-cancer-screening-among-women-nfhs 5- data-reveals-74666. [Last accessed 08 August 2023].
- Tamil Nadu Health Systems Project. Department of Health & Family Welfare, Government of Tamil Nadu. Project overview. Available from: https://tnhsp.org/tnhsp/project.php, [Last accessed 08 August 2023].
- Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: A cluster-randomised trial. Lancet.. 2007;370:398-406.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of VIA screening by primary health workers: randomized controlled study in Mumbai, India. J Natl Cancer Inst.. 2014;106:dju009.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evaluation of adjunctive tests for cervical cancer screening in low resource settings. Indian J Cancer.. 2007;44:51-5.
- [CrossRef] [PubMed] [Google Scholar]
- HPV screening for cervical cancer in rural India. N Engl J Med.. 2009;360:1385-94.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of VIA, Pap, and HPV DNA testing in a cervical cancer screening programme in a peri-urban community in Andhra Pradesh, India. PLoS One. 2010;5:e13711.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Can human papillomavirus DNA testing of self-collected vaginal samples compare with physician-collected cervical samples and cytology for cervical cancer screening in developing countries? Cancer Epidemiol. 2009. ;33:446-50.
- [Google Scholar]
- Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med.. 2018;379:1895-1904.
- [CrossRef] [PubMed] [Google Scholar]
- Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB Squamous squamous cervical cancer: A randomized controlled trial. J Clin Oncol.. 2018;36:1548-1555.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized phase III study comparing neoadjuvant chemotherapy followed by surgery versus chemoradiation in stage IB2-IIB Cervical cervical cancer: EORTC-55994. J Clin Oncol. 2023:JCO2202852.
- [Google Scholar]
- Cancer of the cervix uteri: 2021 update. Int J Gynaecol Obstet.. 2021;155(Suppl 1):28-44.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Variations in gynecologic oncology training in low (LIC) and middle income (MIC) countries (LMICs): Common efforts and challenges. Gynecol Oncol Rep.. 2017;20:9-14.
- [CrossRef] [PubMed] [Google Scholar]
- The need for accredited training in gynaecological oncology: A report from the European network of young gynaecological oncologists (ENYGO) Ann Oncol.. 2013;24:944-52.
- [CrossRef] [PubMed] [Google Scholar]
- Postoperative radiotherapy following inadvertent simple hysterectomy versus radical hysterectomy for cervical carcinoma. Asian Pac J Cancer Prev.. 2011;12:1537-41.
- [PubMed] [Google Scholar]
- Reason for improper simple hysterectomy in invasive cervical cancer in Northeast India. J Cancer Res Ther.. 2022;18:1564-1568.
- [CrossRef] [PubMed] [Google Scholar]
- Results of salvage radiotherapy after inadequate surgery in invasive cervical carcinoma patients: A retrospective analysis. Int J Radiat Oncol Biol Phys.. 2005;63:828-33.
- [CrossRef] [PubMed] [Google Scholar]
- Survival outcome and prognostic factors post inadvertent hysterectomy in carcinoma cervix treated with salvage chemo-radiation. J Cancer Res Clin Oncol.. 2023;149:12355-12364.
- [CrossRef] [PubMed] [Google Scholar]
- The IAEA Directory of Radiotherapy Centres (DIRAC) Available from: , https:/dirac.iaea.org/ [Accessed September 9, 2023].
- Evaluation of socio-demographic factors for non-compliance to treatment in locally advanced cases of cancer cervix in a rural medical college hospital in India. Indian J Palliat Care.. 2013;19:158-165.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240) Lancet.. 2017;390:1654-1663.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol.. 2019;37:1470-1478.
- [CrossRef] [PubMed] [Google Scholar]
- Screening and management of preinvasive lesions of the cervix: Good clinical practice recommendations from the Federation of Obstetrics and Gynaecologic Societies of India (FOGSI) J ObstetGynaecol Res.. 2020;46:201-14.
- [CrossRef] [Google Scholar]
- National cancer grid of India consensus guidelines on the management of cervical cancer. J Glob Oncol.. 2018;787u;4:1-15.
- [PubMed] [Google Scholar]
- Health Technology Assessment of Strategies for Cervical Cancer Screening in India. School of Public Health Postgraduate Institute of Medical Education and Research Chandigarh (India). Available from https://dhr.gov.in/sites/default/files/HTA_CaCx%20Screening%20in%20India.pdf. [Last accessed 10 Aug 2023]
- Programme organization rather than choice of test determines success of cervical cancer screening: Case studies from Bangladesh and India. Int J Gynaecol Obstet.. 2021;152:40-47.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet.. 2014;383:524-32.
- [CrossRef] [PubMed] [Google Scholar]
- The acceptability of self-sampled screening for HPV DNA: A systematic review and meta-analysis. Sex Transm Infect.. 2017;93:56-61.
- [CrossRef] [PubMed] [Google Scholar]
- Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on selfsamples: Updated meta-analyses. BMJ.. 2018;363:k4823.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acceptability and concordance of self- versus clinician- sampling for HPV testing among Rural South Indian Womenwomen. Asian Pac J Cancer Prev.. 2021;22:971-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical evaluation of modifications to a human papillomavirus assay to optimise its utility for cervical cancer screening in low-resource settings: a diagnostic accuracy study. Lancet Glob Health.. 2020;8:e296-304.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evaluation of a chip-based, point-of-care, portable, real-time micro PCR analyzer for the detection of high-risk human papillomavirus in uterine cervix in India. JCO Glob Oncol.. 2020;6:1147-54.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Estimating the value of point-of-care HPV testing in three low- and middle-income countries: a modeling study. BMC Cancer.. 2017;17:791.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- AV Magnivisualizer: a low-cost screening technology for early detection of precancerous and early cancerous lesions of the uterine cervix. BMJ Innovations. 2015;1:99-102.
- [CrossRef] [Google Scholar]
- Is India’s public health care system prepared for cervical cancer screening?: Evaluating facility readiness from the fourth round of the District Level Household and Facility Survey (DLHS-4) Prev Med.. 2020;138:106147.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The current status of palliative care in India - Cancer Control; 2015. Available from: https://www.cancercontrol.info/cc2015/the-current-status-of-palliative-care-in-india/ [Last accessed 31 Aug 2023].
- Practical aspects of palliative care & palliative radiotherapy in incurable cervical cancer. Indian J Med Res. 2021;154:262-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Early integration of palliative care in cervical cancer: Experiences from a pilot study. J Family Med Prim Care.. 2023;12:366-70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Feasibility, acceptability, and efficacy of a community health worker-driven approach to screen hard-to-reach periurban women using self-sampled HPV detection test in India. JCO Glob Oncol.. 2020;6:658-666.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Approaches to recruiting “hard-to-reach” populations into re-search: A review of the literature. Health Promot Perspect.. 2011;1:86-94.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of cervical cancer and associated factors among women attended cervical cancer screening centre at gahandi memorial hospital, Ethiopia. Cancer Inform.. 2021;20:11769351211068431.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cervical cancer screening in HIV-positive women in India: why, when and how? J ObstetGynaecol India. 2021. ;71:304-12.
- [Google Scholar]
- Screening of cervical neoplasia in HIV-infected women in India. AIDS.2013. ;27:607-15.
- [CrossRef] [Google Scholar]
- Screening for early detection of cervical cancer in women living with HIV in Mumbai, India - Retrospective cohort study from a tertiary cancer centre. Available from: https://www.thieme-connect.com/products/ejournals/html/ 10.1055/s-0042-1742662 [Last accessed 23 Jul 2023].
- ConCerv: A prospective trial of conservative surgery for low-risk early-stage cervical cancer. Int J Gynecol Cancer.. 2021;31:1317-1325.
- [CrossRef] [PubMed] [Google Scholar]
- Senticol 2 group. Sentinel lymph node biopsy and morbidity outcomes in early cervical cancer: Results of a multicentre randomised trial (SENTICOL-2) Eur J Cancer.. 2021;148:307-315.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation: a patterns-of-care study. Int J Radiat Oncol Biol Phys.. 1993;25:391-7.
- [CrossRef] [PubMed] [Google Scholar]
- Late toxicity after adjuvant conventional radiation versus image-guided intensity-modulated radiotherapy for cervical cancer (PARCER): A Randomized Controlled Trial. J Clin Oncol.. 2021;39:3682-3692.
- [CrossRef] [PubMed] [Google Scholar]
- The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol.. 2018;9:48-60.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A pilot study of moderately hypo-fractionated whole pelvic radiotherapy with concurrent chemotherapy and image-guided high dose rate brachytherapy for locally advanced cervical carcinoma. Int J Radiat Oncol Biol Phys.. 2022;114:S89.
- [CrossRef] [Google Scholar]
- Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysis. Lancet.. 2001;358:781-6.
- [CrossRef] [PubMed] [Google Scholar]
- Adjuvant chemotherapy following chemoradiotherapy as primary treatment for locally advanced cervical cancer versus chemoradiotherapy alone (OUTBACK): An international, open-label, randomised, phase 3 trial. Lancet Oncol.. 2023;24:468-82.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol.. 2004;22:3113-9.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of tisotumabvedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol.. 2021;22:609-19.
- [CrossRef] [PubMed] [Google Scholar]




