Translate this page into:
NAMS task force report on high altitude
Corresponding author: Lt Gen Sandeep Thareja, SM, VSM**, Director & Commandant, Armed Forces Medical College, Pune, India. sandeepthareja@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Thareja S, Chandra BA, Aggarwal V, Uday Y, Shekhar A, Singh K, et al. NAMS task force report on high altitude. Ann Natl Acad Med Sci (India) 2025;61:45-65. doi: 10.25259/ANAMS_TFR_08_2024
INTRODUCTION
High-altitude (HA) medicine, also known as high-altitude physiology or mountain medicine, is a specialized field of medicine that focuses on the effects of high altitudes on the human body and the prevention and treatment of altitude-related illnesses. As more people venture to high-altitude regions for work, recreation, and travel, the importance of research in this field becomes increasingly evident. Here are some key points that highlight the significance of high-altitude medicine and the need for ongoing research:
Growing tourism and travel: High-altitude destinations such as the Andes, Himalayas, and the Rocky Mountains attract millions of tourists and adventurers every year. Understanding the health risks associated with high altitudes and developing effective strategies for prevention and treatment are essential to ensure their safety.
Healthcare in remote areas: Many high-altitude regions have remote access to medical facilities. Research in high-altitude medicine can lead to the development of better healthcare protocols and technologies for these areas.
Climate change and altitude: Climate change is affecting high-altitude regions, leading to shifts in weather patterns, glacial melting, and altered ecosystems. Research is needed to understand how these changes impact human health and adaptation to high altitudes.
Occupational health: Certain occupations, such as mining, military, and research, require people to work at high altitudes. Occupational health research can help ensure the safety and well-being of these workers.
Altitude-related illnesses: Altitude sickness, acute mountain sickness (AMS), high-altitude pulmonary edema (HAPE), and high-altitude cerebral edema (HACE) are common altitude-related illnesses. Research can provide insights into the causes, risk factors, and effective treatments for these conditions.
Physiological adaptations: Understanding how the human body adapts to high-altitude environments can have broader implications for medicine and physiology. Research in this field can shed light on respiratory and cardiovascular adaptations, which may have applications beyond high-altitude settings.
Preventive measures: Developing effective preventive measures, such as gradual acclimatization, pharmacological interventions, and oxygen supplementation, relies on ongoing research to refine and optimize these strategies.
Aging population: As the global population ages, more older individuals may be exposed to high altitudes. Research is needed to understand how aging affects susceptibility to altitude-related illnesses and the efficacy of treatments in this demographic.
Genetic factors: Genetic predisposition can influence an individual’s response to high altitudes. Research can help identify genetic markers associated with altitude-related illnesses and potentially lead to personalized preventive and treatment strategies.
International collaboration: Many high-altitude regions span multiple countries. International collaboration in high-altitude medicine research can lead to the development of standardized guidelines and best practices for healthcare in these regions.
Hence, high-altitude medicine is a vital field with growing relevance due to increased travel, climate change, and occupational exposure. Continued research is essential to improve our understanding of altitude-related health issues and to develop better strategies for the prevention and treatment of altitude-related illnesses, ultimately ensuring the safety and well-being of individuals who visit or work in high-altitude areas.
DEFINITIONS
High altitude: HA is defined as an altitude above 2700 m (9000 feet). Ascent to these altitudes is associated with a significant risk of acute and sub-acute/chronic high altitude illness (HAI). Above this altitude, there is a definite and significant reduction in peak exercise capacity and sub-maximal exercise endurance. This is so since, at this altitude, the low ambient barometric pressure results in an alveolar oxygen partial pressure (PAO2) close to 66 mmHg. At this PAO2, the effects of hypoxia on the human body are obvious and easily recognizable.
Extreme altitude: Altitudes greater than 5500–5800 m (18,000–19,000 feet) are classified as extreme altitude (EA). Ascent to these altitudes is associated with a greater risk of acute and sub- acute/chronic HAI. The physiological response to these altitudes is pronounced as is decrement in maximal exercise capacity and endurance for submaximal exercise. The degree and time course of acclimatization to these altitudes is debatable, and it is believed that the human body does not completely acclimatize to extreme altitudes.
Moderate altitude: Altitudes between 1500 and 2700 m (5000–9000 ft) are classified as moderate altitudes. Certain physiological functions, such as exercise capacity are impaired at these altitudes, and a definite acclimatization response has been reported. Acute HAIs are known to occur at these altitudes; however, their incidence is very low.
ALTITUDE RELATED MEDICAL PROBLEMS
All ailments that affect humans at sea level may affect them at HA also. HAI, however, refers to a set of ailments unique to HA, with hypobaric hypoxia as the central etiological factor. Several other medical problems may also occur at HA that are not specific to HA but are exacerbated or precipitated by the HA environment. Currently, there is no universally accepted classification scheme for these ailments, and this article has grouped them as miscellaneous medical problems at HA and altitude-exacerbated conditions [Table 1].
High altitude illness: HAI or disease refers to clinical syndromes that occur as a consequence of exposure to the HA environment, with hypobaric hypoxia being the central etiological factor. HAI are classified based on their onset time [Table 1]. Acute illnesses occur within hours to days of ascent to HA, subacute within weeks to months, and chronic after months to years of stay at HA. Ascent to HA often produces symptoms such as breathlessness on exertion and palpitations in healthy individuals. These are often the result of the normal physiological responses of the body to hypobaric hypoxia and should not be confused with acute HAI.
Risk factors for HAI: Several factors may predispose an individual to HAI [Table 2]. Faster rates of ascent, greater sleeping altitude, and physical activity early after ascent to HA are important predisposing factors for acute HAI. The altitude of residence, previous history of HAI, and individual susceptibility are other factors that contribute to the risk of acute and chronic HAI. Acute HAI occurs less in the elderly (probably due to less exertion at HA), while women are known to suffer lesser HAPE than men. This may be an observational bias because a far lesser number of women ascend to HA when compared to men. Physically fitter individuals are believed to suffer a greater incidence of acute HAI. However, once the risk of acute HAI is mitigated, those who continue to stay at HA physically perform better. Obesity has been implicated as a risk factor for AMS. Underlying cardiorespiratory disorders that lead to greater pulmonary arterial pressure (PAP) and concurrent infections of the upper respiratory tract are known to increase the risk of HAPE.
|
HA: High altitude, COPD: Chronic obstructive pulmonary disease
|
|
|
|
|
|
|
|
|
|
HA: High altitude
ACCLIMATIZATION
Introduction: The HA environment exposes us to many stressors, principal among which are hypobaric hypoxia, cold, low humidity, and increased ultraviolet (UV) radiation [Table 3]. The most important stressor by far is hypobaric hypoxia. This induces a marked systemic response, introduces the risk of illnesses unique to HA, and reduces individuals’ physical work capacity and endurance for the duration of stay. Sojourn at HA may also aggravate pre-existing diseases such as coronary artery disease, bronchial asthma, and thrombophilia.
|
|
|
|
PO2: Partial pressure of oxygen, UV: Ultraviolet
Any of the stressors listed above could be a cause of illness at HA. Illnesses attributable to a direct effect of hypobaric hypoxia are called HAI, and based on the duration of stay and occurrence, they are classified as Acute HAI, for example, AMS, sub-acute HAI, for example, high altitude pulmonary hypertension (HAPH) (may also occur in a chronic form), and chronic HAI, for example, chronic mountain sickness (CMS). Cold exposure may lead to chilblains and frostbite, low humidity may lead to dehydration during activity, and UV radiation frequently causes dermatitis at HA. These along with other illnesses, are discussed in detail in the section on altitude-related medical problems.
The human body responds to HA by certain systemic changes starting immediately on the ascent and continuing over hours, days, weeks, and months. This response helps us to live and perform better at HA and is known as “acclimatization.” Acclimatization is reversible upon descent to lower altitudes.
Continuing acclimatization at a given altitude will lead to:
Reduced risk of acute HAI at the altitude
Reduced risk of acute HAI on further ascent
Improved individual work endurance, however, the peak work capacity never recovers to sea level values.
The rate and magnitude of the acclimatization response depends on the rate of ascent to HA and the actual altitude attained. There is considerable variability in these responses between different individuals. The failure of adequate acclimatization responses or an exaggerated response can both lead to HAI. For example, a blunted ventilatory response to hypoxia or an exaggerated rise in hemoglobin at HA can predispose an individual to AMS and CMS, respectively. The time course of the reversal of changes in acclimatization is not well documented but may take hours to days, weeks, and maybe months. For example, the upregulated hypoxic ventilatory response seen with a stay at HA may reverse in hours to days, whereas the increased hemoglobin values and hematocrit seen at HA, in all probability, takes over weeks to months to decline.
THE PHYSIOLOGY OF ACCLIMATIZATION
Hypoxia of high altitude: Barometric pressure falls with increasing altitude. This fall is, however, nonlinear, with a more rapid fall near the surface of the earth than at relatively higher altitudes. Dalton’s Law of partial pressure states that every gas in a mixture of gasses exerts a pressure proportional to its concentration in the gas mixture. At sea level, the partial pressure of oxygen in ambient air is 159 mmHg. At 9000 ft, partial pressure would be 116 mm Hg. Thus, at high altitudes, the PAO2 in ambient air and, consequently, in inspired and alveolar air is reduced. Since the oxygen partial pressure in the alveoli is the driving force for oxygen diffusion from the alveolus into pulmonary capillary blood, the reduced PAO2 leads to a lowered PAO2 in arterial blood, and this translates finally to lowered oxygen delivery to tissues, resulting in tissue hypoxia. Table 4 shows changes in alveolar gas compositions at high altitudes.
| Altitude |
Barometric pressure (mm Hg) |
PO2 in ambient air (mm Hg) |
PO2 in alveolar air (mm Hg) Without ventilatory acclimatization |
PO2 in alveolar air (mm Hg) With ventilatory acclimatization |
|---|---|---|---|---|
| Sea level | 760 | 159 | 104 | NA |
| 11,300 ft (altitude of Leh) | 510 | 106 | 47 | 57 |
| 18,000 ft | 390 | 81 | 31 | 50 |
| 29,029 ft (altitude of Mt Everest) | 253 | 53 | -5 | 34 |
Note: The barometric pressure values are measured at locations in Ladakh, India, during September (except for the value at 29,029 ft). These would vary with season, greater in winter and lesser in summer. The PAO2 is calculated by the alveolar gas equation with and without ventilatory acclimatization. PACO2 values of 40, 32, 26, and 7.5 mmHg have been assumed for the ventilatory acclimatized state.
PO2: Partial pressure of oxygen, PAO2: Partial pressure of oxygen in alveolar air, PACO2: Partial pressure of carbon dioxide
The physiological changes that constitute acclimatization are responses of the human body to the hypobaric hypoxia of high altitude. Three physiological systems play a predominant role in altitude acclimatization. These are:
The respiratory system
The hematological system
The cardiovascular system
RESPIRATORY CHANGES AT HIGH ALTITUDE
Hyperventilation: The hypobaric hypoxia of HA causes a fall in the PAO2 in arterial blood. This leads to the stimulation of the peripheral chemoreceptors in the carotid and aortic bodies, which results in hyperventilation. The hyperventilatory response is termed as the “Hypoxic ventilatory response” (HVR) and occurs within minutes of arrival at a high altitude. The magnitude of this hyperventilatory response depends on the rate of onset and magnitude of hypoxia. The initial increase in ventilation is largely due to an increase in tidal volume and the subsequent increase in the rate of respiration. The magnitude of HVR varies from individual to individual, and it has been suggested to correlate positively with physical performance early after arrival at HA and inversely with susceptibility to AMS. The hyperventilation lowers the PACO2, and this factor contributes to raising the alveolar PAO2 by a few millimeters of Hg. The respiratory alkalosis as a result of HVR is partially corrected by a compensatory excretion of bicarbonate by the kidney over the next 48–72 hours, and respiration stabilizes over the next few days to weeks (depending on the altitude) at a new functional level, higher than that at sea level. The respiratory alkalosis at HA has important consequences on transport and tissue delivery of O2 by blood.
Hemoglobin-oxygen (Hb-O2) dissociation curve: The Hb-O2 dissociation curve is sigmoid shaped. At a PAO2 greater than 60 mmHg, the saturation of Hb with O2 is greater than 90% (flat upper part of the curve). However, once the PAO2 falls below 60 mm Hg, a small fall in PAO2 causes a large fall in the saturation of Hb with O2 (steep part of the curve). The respiratory alkalosis at high altitude stimulates the formation of 2-3 Diphosphoglycerate (2-3 DPG) within the red blood cells. Since alkalosis and 2-3 DPG exert opposing influence on the Hb-O2 dissociation curve, the curve is not significantly affected at HA. However, with a further increase in altitude, the effect of alkalosis predominates, leading to a left shift of the curve. This facilitates O2 uptake in the lungs at low PO2 of air.
Hypoxic pulmonary vasoconstriction (HPV): The pulmonary vasculature constricts in response to hypoxia and results in elevated pulmonary arterial pressures. This response is uneven in the pulmonary vascular bed with some areas showing greater vasoconstriction when compared to others. The heterogeneity of vasoconstriction reflect the inherent differences in the ventilation of different lung segments. Those segments with lower ventilation are likely to exhibit greater vasoconstriction when compared to better-ventilated segments. The magnitude of HPV varies in different individuals as does the resultant elevation of the PAP. The uneven HPV and raised PAP at HA are important factors underlying the development of HAPE. Factors such as exercise and low environmental temperatures aggravate the rise in pulmonary arterial pressures and increase the risk of HAPE.
HEMATOLOGICAL CHANGES AT HIGH ALTITUDE
An acute rise in the hematocrit is often seen during the first few days after arrival at a high altitude. This is the result of hemoconcentration that occurs due to (a) plasma volume shrinkage due to increased fluid loss compared to fluid intake and (b) redistribution of fluid from the intravascular to extravascular compartment in the initial days at HA.
Hemoglobin, hematocrit, and blood volume: Hypoxia is a potent stimulus for increased erythropoiesis. The effects of the erythropoietic response begin to manifest in about 3–4 days and probably reach a maximum after about 3 weeks of stay at HA. This response may last for months depending on the altitude of stay and an individual’s erythropoietic response. At EA, this may lead to the expansion of the total blood volume and hyperviscosity. The increased hemoglobin concentration offsets the effect of decreased saturation at HA by increasing oxygen content per volume of blood. This, along with the increased cardiac output at HA allows the blood–tissue O2 gradient to be maintained and tissue extraction of O2 to be achieved without a significant fall in venous oxygen tension.
CARDIOVASCULAR CHANGES AT HIGH ALTITUDE
Cardiac output: The hypoxic environment of high altitude is a challenge for adequate oxygen delivery to tissues. The cardiovascular system responds to this challenge by a central activation of the sympathetic nervous system and the compensatory changes in the local tissue blood flow regulatory mechanisms. The cardiac output is seen to increase during acute ascent to HA. This increase is principally due to an increase in the heart rate. The magnitude of the increase in cardiac output depends on the altitude. A 40% increase in the resting cardiac output has been reported following acute ascent to altitudes between 3600 and 4250 m (12,000–14,000 ft), although increases to the tune of 75% have been reported on acute ascents to altitudes greater than 4500 m (15,000 ft). With acclimatization, the resting cardiac output approaches near sea-level values, but the cardiac output during exercise is lower than at sea level.
Peripheral vascular resistance (PVR) and blood pressure: During the first few hours of ascent to HA, the systemic arterial blood pressure remains unchanged or might be slightly lower than sea level values. It is then often seen to rise over the next few weeks. This alternating trend of systemic arterial blood pressure is due to the opposing influences of increased sympathetic activity (leading to increased PVR and raised blood pressure) and hypoxia-induced vasodilatation in tissue beds (leading to decreased PVR and lowered blood pressure). Certain individuals, however, do not show a reduction of systemic arterial blood pressure at HA. The exact mechanism responsible for changes in systemic arterial blood pressure at HA and reasons for individual variation in responses are still unclear and are an important area of research.
OTHER IMPORTANT PHYSIOLOGICAL ALTERATIONS AT HIGH ALTITUDE AREA (HAA)
Physical performance at high altitude: Physical work capacity, peak as well as endurance for sub-maximal exercise, reduces with an increase in altitude beyond 1500 m, (5000 ft). There is an approximate 11% reduction in maximum oxygen consumption (VO2max) per 1000 m (3280 m) gain in altitudes above 1500 m. Acclimatization causes an improvement in the VO2max and endurance for submaximal exercise, but individuals do not attain sealevel values while at HA. The VO2max has been reported as being 85% of the sea level value at 3000 m, (10,000 ft) 60% of the sea level value at 5000 m (16,500 ft) and 20% of the sea level at 8848 m (29,021 ft).
Note: The physiological changes that constitute altitude acclimatization are specific to that altitude. Any further ascent of more than 500 m (approx. 1600 ft) by individuals acclimatized to a particular altitude would render them susceptible to HAI until they acclimatize to the new altitude.
ACUTE MOUNTAIN SICKNESS (AMS)
Definition and epidemiology: AMS is a syndrome of nonspecific symptoms and is defined as the occurrence of headache along with the presence of one or more of the following: gastrointestinal symptoms, dizziness, lassitude, or fatigue in an unacclimatized individual, usually within 3 days of arrival at HA. The incidence of AMS varies with the altitude and rapidity of ascent and ranges from 3.1% at 2000 m (7000 ft) to 53% at 5000 m (16,000 ft).1
Etiopathogenesis: The exact pathophysiological mechanisms responsible for AMS are yet to be established. The critical factor in the pathogenesis of AMS is hypoxemia. It is postulated that AMS may represent the benign end of a clinical spectrum, with the malignant end being HACE. Evidence of cerebral edema and raised intracranial tension in AMS is, however, inconsistent. Some other postulated pathophysiological mechanisms include hypoxia-mediated release of vasogenic substances, activation of the trigeminovascular system, and free radical–mediated alteration of the blood brain barrier permeability. Hypobaria may contribute to AMS by causing blunting of ventilation, impaired lymphatic drainage from the lung, and changes in autonomic functions. Current evidence does not support the role of generalized fluid retention in the pathogenesis of AMS.2
Clinical features: The predominant symptom of AMS is headache, which is generally frontal, throbbing, aggravated by exertion and more severe in the mornings. It may be accompanied by malaise, giddiness, anorexia, nausea, and vomiting. Symptoms of AMS typically develop within 6–10 hours after ascent to HA, sometimes as early as within 1 hour, but invariably within the first 3 days of ascent to HA. The greater the ascent, earlier the onset of symptoms; predisposed individuals may also develop symptoms earlier. Usually, AMS is worst after the first night of sleep at a given altitude. Recovery usually occurs within 72 hours with rest and no further gain in altitude. In rare instances, the headache or another symptom of AMS may persist for weeks after arrival at HA. The onset of symptoms after 3 days of arrival at HA or failure to respond to oxygen or descent should raise the suspicion of alternative diagnosis.
-
Diagnosis: AMS is a clinical diagnosis. A commonly used tool to diagnose AMS is the lake louise scoring system (LLS-2018),3 which comprises a self-reported questionnaire of symptoms consisting of headache, gastrointestinal symptoms, fatigue, and dizziness. The symptoms are rated in severity on a scale of 0–3. (Appendix A). A self-reported score greater than or equal to 3 suggests AMS. Self-reported score of 3–5 implies mild AMS, and score “6” implies moderate to severe AMS. It is apparent that any illness with symptoms similar to AMS will form a differential diagnosis of AMS. Thus, the decision to treat as AMS must be guided by the occurrence of symptoms in the “setting of a recent gain in altitude” and the exclusion of other likely causes of the symptoms [Table 5]. Conditions such as dehydration and fatigue after a long trek also involving ascent often mimic AMS. When uncertain of the diagnosis, it may be good to treat as AMS until proven otherwise.
Table 5: Differential diagnosis of AMS/HACEDehydration
Exhaustion
Hypoglycemia
Diabetic ketoacidosis
Hyponatremia
Hypothermia
Carbon-monoxide poisoning
Migraine, High altitude headache
Cerebrovascular spasm
CNS infections
Acute psychosis
Stroke/TIA
CNS tumor
Ingestion of drugs, alcohol, or toxins
Seizure disorder
AMS: Acute mountain sickness, HACE: High altitude cerebral edema, TIA: Transient ischemic attack, CNS: Central nervous system
Treatment: The treatment of AMS consists of treatment for relief of symptoms such as headache and nausea and specific treatment to correct the underlying pathophysiological mechanisms responsible for the condition. Symptomatic treatment should be started even if the LLS score is below 3. An algorithm for the management of AMS in the resource-poor or periphery setting is shown in Figure 1.
Treatment of AMS in an institutional setting: Symptomatic therapy is advisable for all cases of mild AMS and comprises nonsteroidal anti-inflammatory drugs for the relief of headaches and antiemetics for the treatment of nausea. Oxygen must be administered at the rate of 1–2 L/min via nasal prongs for 12–24 h, and this generally leads to the resolution of symptoms. In cases of moderate to severe AMS or if no relief with the above treatment, the specific treatment for AMS comprises Acetazolamide tablet in a dose of 250 mg 12-hourly or 125 mg 8-hourly. Tablet Dexamethasone 4 mg 6-hourly is a useful alternative in moderate AMS and may be used in patients with sulfonamide sensitivity. Supportive measures include rest and avoidance of exertion, avoidance of alcohol and sedatives, and ensuring adequate fluid intake. Reascent may be allowed on the complete resolution of symptoms.
-
Complications: AMS may progress to HACE in rare cases if not promptly managed or if the hypoxemia is worsened with physical exertion and/or a further gain in altitude. The appearance of change in mental status and/or ataxia indicates onset of HACE and must be managed accordingly.
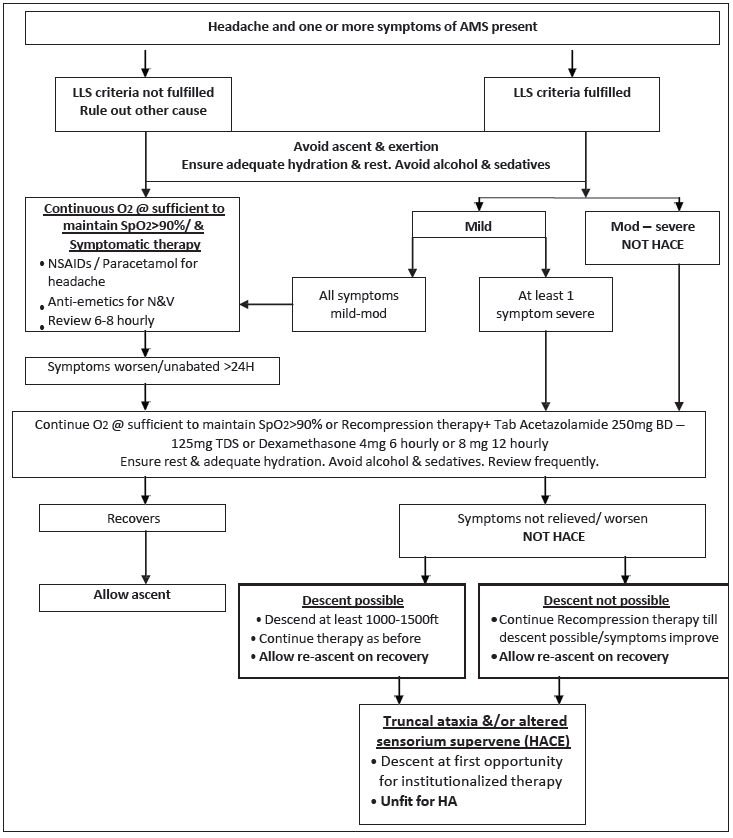 Figure 1:
Figure 1:- Algorithm for treatment of AMS in the peripheral setting. AMS: Acute mountain sickness, LLS: Lake Louise acute mountain sickness score, NSAIDs: Nonsteroidal anti-inflammatory drugs, N&V: Nausea and vomiting, HACE: High altitude cerebral edema, HA: High altitude
Prognosis: AMS is usually a benign, self-limiting condition that recovers spontaneously within 48–72 hours of onset; most cases subsides with rest and cessation of ascent. Once recovered, the individuals may be allowed to ascend. Usually, symptomatic therapy is all that is required; moderate to severe cases require specific therapy but may ascend after recovery.
-
Prevention: The options available for the prevention of AMS, and also for acute HAI, are as follows:
Staged ascent: A staged ascent is an excellent method to reduce the incidence and severity of AMS. Staged ascent allows time for the body for sufficient acclimatization to prevent acute HAI. A number of staging protocols exist and are tailored to topographical and logistic requirements and to the availability of time. The staging schedule used by the Indian Army has been proven to definitely lower the incidence and severity of AMS [Table 6].4
-
Drug prophylaxis: Is advisable when staged ascent may not be an option or an individual is known to be prone to AMS.
Table 6: Recommended rates of ascent at high altitudeAbove 2700 m altitude: Gain in sleeping altitude should not exceed 500 m per day
Allow one rest day for every 1000–1500 m gain in altitude
Do not ascend if symptoms of AMS appear. Ascend only after symptoms completely regress
Descend to lower altitude if symptoms persist or deteriorate.
AMS: Acute mountain sickness
Tablet Acetazolamide (125 mg 12-hourly or 250 mg sustained release preparation once a day) started 24 hours prior to the ascent of HA and continued for 3 days at HA is the drug of choice for prevention of AMS.
-
Tablet Dexamethasone (2 mg 6-hourly/4 mg 12-hourly) reduces the incidence and severity of AMS. It has the additional advantage of enhancing exercise capacity at HA for the duration of use. It may be used in individuals.
Known to be sulphonamide-sensitive
Where immediate activity is required after sudden ascent, for example, airlift to 3300 m with immediate need for activity.
Dexamethasone is known to interfere with the normal acclimatization process and a rebound increase in AMS may occur on discontinuing the drug at HA, especially in individuals engaged in physical activity. The acute adverse effects of steroid therapy, such as euphoria and disorientation, must be borne in mind when using Dexamethasone.
Pre-acclimatization: Stay at moderate altitudes of 1800 m (5000–6000 ft) for a period of 2–8 months has been reported to provide a definite degree of acclimatization and reduce the severity and incidence of AMS on further ascent.
HIGH ALTITUDE CEREBRAL EDEMA (HACE)
Definition and epidemiology: HACE is a potentially fatal condition that is diagnosed clinically as per the LakeLouise consensus criteria as the presence of ataxia and/or altered consciousness in an individual who may or may not have AMS/HAPE. HACE can present within 3 to 5 days of arrival to elevations as low as 2700 m (9000 ft). HACE may occur within a shorter time frame during rapid ascent to greater altitudes, and thus, the features of preceding AMS may be masked and absent. The incidence of HACE varies principally with the rate of ascent and the sleeping altitude attained during a particular ascent. It has been reported as less than 0.1% in studies. Up to 14% of cases of HAPE are known to have concomitant HACE, probably a result of the accentuated hypoxia consequent to HAPE.1,2
Etiopathogenesis: The exact pathophysiological basis of HACE is not well established. Some of the postulated mechanisms are increased capillary permeability coupled with increased cerebral blood flow and capillary hydrostatic pressure seen in the early days at HA. It is also hypothesized that fluid retention may have a role to play in the development of HACE.
Clinical features: Altered mental status and truncal ataxia are the diagnostic clinical features of HACE. The tandem gait test is the best test for evaluating this; HACE does not affect finger-to-nose tests for ataxia. Mental status changes may range from irrational behavior progressing rapidly to lethargy, depressed sensorium, hallucinations, and coma. Associated findings may include papilledema, retinal hemorrhages, cranial nerve palsies, and abnormal reflexes. Focal neurological deficit is usually rare.
-
Diagnosis: HACE is diagnosed clinically. The presence of altered mental status or ataxia in an individual showing features of AMS or the presence of an altered mental status AND ataxia in the absence of features of AMS, in individuals within 3–5 days of ascent to HA is HACE unless proved otherwise. A number of conditions can mimic HACE/AMS [Table 5], and the evidence for these must be sought where features of AMS/HACE occur in atypical settings, that is, no history of recent gain in altitude, absence of a precipitating factors such as unusual exertion, and failure to respond to therapy or unusually rapid response to therapy.3
Where computed tomography (CT) imaging is available in severe HACE, it may reveal nonspecific findings of diffuse cerebral edema, such as the absence of sulci, small ventricles, and a diffuse low-density appearance of the entire cerebrum [Figure 2]. Diffusion-weighted MRI is the most reliable diagnostic modality available. T2-weighted MRI reveals reversible white matter edema, especially in the splenium of the corpus callosum and the centrum semiovale, without involvement of the gray matter.
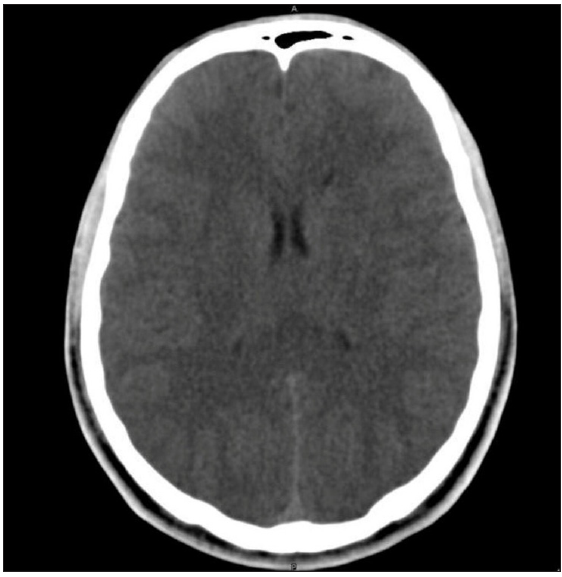 Figure 2:
Figure 2:- CT Scan in a patient of HACE showing absence of sulci, small ventricles, and a diffuse low-density appearance of the entire cerebrum. HACE: High-altitude cerebral edema. CT: Computed tomography
Table 7: Guidelines for the management of high altitude cerebral edema (HACE) in the resource limited or peripheral setting-
Do not worsen the hypoxia
No physical exertion/further gain in altitude
Correct the hypoxia
Immediate descent to lower altitude
Actual descent to lower altitude
Simulated descent using recompression chambers till actual descent possible
Supplemental oxygen via facemask at 2–4 L/min
Pharmacotherapy
Dexamethasone 8 mg initially orally/IM/IV followed by 4 mg every 6 hours.
Tablet Acetazolamide 250 mg 12-hourly if descent to lower altitude is delayed
Injection Mannitol/Glycerol to reduce intracranial pressure
IM: Intramuscular, IV: Intravenous
-
Treatment in the resource poor or peripheral setting: HACE is a medical emergency requiring immediate descent to a lower altitude. Symptoms are typically seen to resolve with a descent of 300–1000 m (1000–3300 ft). Where actual descent is not possible, simulated descent using recompression chambers is lifesaving. Portable recompression chambers can generate pressures up to 130 mmHg, simulating a descent of about 6000 feet. Supplemental oxygen sufficient to raise the arterial oxygen saturation (SaO2) to >90% should be administered where descent to lower altitudes is not possible. Oral or parenteral Dexamethasone (8 mg stat followed by 4 mg 6-hourly) and tablet Acetazolamide (250 mg 12-hourly) should be started if available and continued till symptoms resolve [Table 7].5
Institutionalized care: Therapeutic measures at a hospital include supplemental oxygen, decongestive or measures to lower intracranial pressures, hyperbaric therapy (if available), and other supportive measures for care of a comatose patient. Oxygen should be given by a face mask (maintaining SaO2 > 90%), or a recompression chamber may be used to simulate a descent to sea level, if available. Decongestive measures should be simultaneously started, including parenteral steroids (Dexamethasone 8–10 mg IV, IM, or PO, followed by 4 mg every 6 h), intravenous Mannitol, and oral glycerol. Attempting to decrease intracranial pressure by intubation and hyperventilation might be tried in refractory cases. Diuretics have not been studied systematically for the treatment of HACE but are reasonable, especially in a hospital setting with care to avoid hypotension. Furosemide may be given in small doses. Treatment of concomitant HAPE with nifedipine may lower systemic arterial blood pressure and cerebral perfusion pressure. This drug should be used cautiously and with frequent monitoring of the blood pressure.
Complications: HACE can be fatal if not managed as a medical emergency.
Prognosis and prevention: HACE is a life-threatening condition. The mortality may be as high as 25% even with prompt institution of therapy. Prevention of HACE is achieved by the same means as for AMS.
Employability restrictions: A patient who develops HACE is unfit for re- employment to HA as she/he is at a high risk of development of a potentially life threatening condition on exposure to HA.
HIGH ALTITUDE PULMONARY EDEMA (HAPE)
Definition and epidemiology: HAPE is a noncardiogenic pulmonary edema characterized by pulmonary hypertension, leading to extravasation of fluid from the intravascular compartment in the lungs of healthy individuals with no underlying cardiac or pulmonary disease. HAPE usually occurs on the second or third day of arrival at HA and rarely after the fourth day. The incidence varies from 0.125 to 15%, being higher with faster rates of ascent to greater altitudes.
Etiopathogenesis: Elevated PAP due to hypoxic pulmonary vasoconstriction is critical in the pathogenesis of HAPE. The nonhomogeneous pulmonary vasoconstriction results in areas of hyper-perfusion, leading to a stress failure of the pulmonary endothelium and extravasation of protein-rich fluid into the pulmonary interstitium and alveoli. Exposure of the basement membrane to the protein-rich edema fluid results in secondary inflammation, activation of coagulation, and generation of microthrombi in the lungs. Preexisting inflammation, for example, preexisting viral infection, may make the endothelium more prone to disruption. Vigorous physical exertion in unacclimatized individuals and exposure to low environmental temperatures may precipitate or aggravate HAPE by raising the PAP. An exaggerated rise of pulmonary artery pressure in response to alveolar hypoxia, increased sympathetic activity, lower endogenous nitric oxide production, and lower rates of alveolar fluid clearance are other factors that may predispose certain individuals to HAPE.1
Clinical features: Dry cough and reduced physical performance for the given altitude in an individual, usually within 3–5 days of arrival at HA, suggest HAPE. Rarely, HAPE may occur in an “acclimatized” individual after weeks to months of stay at HA. However, a precipitating factor, for example, unusually severe exertion or gain in altitude, is invariably present. Other common symptoms on presentation are dyspnea, chest discomfort, and fatigue or weakness. Pink, frothy sputum and respiratory distress occur later in the illness. Orthopnea and hemoptysis are uncommon. Patients usually show resting tachycardia and tachypnoea that become pronounced as the illness progresses. Crackles and wheeze are usually present in more than one lung field.
Diagnosis: HAPE is diagnosed clinically as the presence of any two of the symptoms of cough, chest discomfort, dyspnea, and fatigue and any two signs of crackles or wheeze on auscultation of the chest, central cyanosis, tachycardia, and tachypnea [Table 8],5 usually within 3–5 days of arrival at HA. Investigations may reveal the following:
ECG: sinus tachycardia, right ventricular strain, right axis deviation, right bundle branch block (RBBB) and P wave abnormalities
-
X-ray chest: Radiographic findings are variable but usually reveal characteristic patchy infiltrates and lack of cardiomegaly or Kerley B lines. Involvement is usually bilateral, but if unilateral, infiltrates are common in the right middle lung field. The pulmonary arteries are usually dilated. Resolution of lung opacities occurs quickly with treatment and lags behind, only briefly, the signs of clinical improvement [Figure 3].
Table 8: Clinical criteria for diagnosing High Altitude Pulmonary EdemaHistory of recent gain in altitude along with any two of the following symptoms:
Cough
Dyspnea at rest
Chest discomfort
Weakness/fatigue
AND Any two of the following signs:
Crackles/wheeze in at least one lung field
Central cyanosis
Tachycardia
Tachypnea
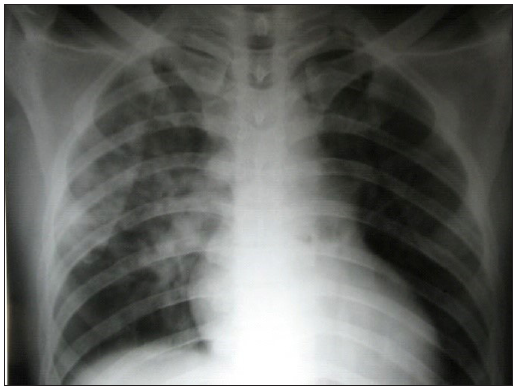 Figure 3:
Figure 3:- Chest X-ray in a patient of HAPE showing a normal cardiac shadow, characteristic patchy irregular infiltrates in the right middle lung fields with sparing of the apices and areas above the diaphragm. HAPE: High altitude pulmonary edema.
Table 9: Differential diagnosis of HAPEBronchial asthma
Acute bronchitis
Pneumonia
Congestive cardiac failure
Pulmonary embolism
Myocardial infarction
Hyperventilation syndrome
Pneumothorax
HAPE: High altitude pulmonary edema
Note: Occurrence of symptoms after 3–5 days of arrival at HA is unusual, and alternative diagnoses such as pneumonia, cardiogenic pulmonary edema, pulmonary embolism, and spontaneous pneumothorax should be considered [Table 9].
Severity classification. HAPE is classified into mild, moderate, serious, and severe based on clinical symptoms, magnitude of tachycardia and tachypnea, and findings on Chest radiograph [Table 10].6
Treatment in resource-limited setting or periphery: Descent to a lower altitude is the first treatment priority for patients of HAPE.7 A descent of at least 1000 m (3300 ft) or till an altitude where symptoms resolve is ideal. In case actual descent is not possible, simulated descent using portable recompression chambers and supplemental oxygen should be administered [Table 11].5 Nifedipine in a dose oof 30 mg sustained release (SR) every 12 hours or 20 mg (SR) every 8 hours should be started in case the above treatment modalities are not possible or if there is no resolution or worsening of symptoms.
-
Institutionalized care: Supplemental oxygen is the mainstay of therapy. Oxygen is provided by a facemask at a rate sufficient to maintain SaO2 > 90%, until symptoms improve and tachycardia subsides. Elevated PAP usually reduces with the correction of hypoxemia. continuous positive airway pressure (CPAP) can be considered as an adjunct to supplemental oxygen, and nifedipine can be added if the patient fails to respond to oxygen therapy alone. There is no role for the use of diuretics, Acetazolamide, and beta-agonists in the treatment of HAPE. Supportive measures like warmth, rest, and adequate hydration should be ensured.
Table 10: Classification of HAPE based on severityGrade Symptoms Heart rate
(beats/min)
Respiratory
rate (breaths/min)
Chest X-ray Mild Dyspnea on moderate exertion
Able to perform light activity
<110 <20 Minor opacities involving < ¼ of one lung field Moderate Dyspnea at rest
Weakness, fatigue on slight effort, cannot perform light activity
Headache with cough
110–120 20–30 Opacities involving at least ½ of one lung field Serious Severe dyspnea,
Loose recurrent productive cough,
Wheezy, difficult respiration, Obvious cyanosis,
Weakness headache, nausea at rest
121–140 31–40 Opacities involving at least ½ of each lung field or unilateral exudates involving all of one lung field Severe Clouded consciousness, stupor, or coma
Unable to stand or walk, severe cyanosis, bubbling rales
Copious bloody sputum
Severe respiratory distress
>140 >40 Bilateral opacities involving > ½ of each lung field HAPE: High altitude pulmonary edema
Table 11: Guidelines for management of HAPE in resource limited settingDo not worsen the hypoxia
No physical exertion
Correct the hypoxia
Descend to lower altitude*
Actual descent where possible
Simulated descent using recompression chamber
Supplemental oxygen via face mask @ 2–4 L/min (if available)
Pharmacotherapy to lower pulmonary artery pressure#
Nifedipine 30 mg SR 12-hourly/20 mg SR 8-hourly.
* All cases of HAPE must be evacuated to hospitals at lower altitudes.#If evacuation to a lower altitude is not immediately possible or the patient shows worsening of symptoms, nifedipine should be administered as an adjunct to simulated descent and oxygen therapy while monitoring the patient’s blood pressure. The drug should not be administered if there is a suspicion of acute myocardial infarction or congestive cardiac failure. HAPE: High altitude pulmonary edema. Complications: HAPE can be fatal if not recognized and treated promptly. Up to 14% of patients with HAPE may show features consistent with HACE, although hypoxic encephalopathy may have the same features.
Prognosis and prevention: HAPE is eminently preventable and has a mortality of <1% if diagnosed and managed promptly. The mainstay of prevention is a staged or gradual ascent to HA. Drug prophylaxis for the prevention of HAPE should only be considered for individuals with a prior history of HAPE. Nifedipine in a dose of 30 mg (SR) 12-hourly or 20 mg (SR) 8-hourly is the recommended drug for HAPE prevention.
SUB-ACUTE AND CHRONIC HA ILLNESSES
A) High altitude pulmonary hypertension (HAPH)
Definition and epidemiology: HAPH is a clinical syndrome that occurs in permanent residents of altitudes > 2500 m (8000 ft) and is characterized by a raised systolic PAP >50 mmHg or a raised mean PAP > 30 mmHg, right ventricular hypertrophy, cardiac failure, moderate hypoxemia, and absence of excessive erythrocytosis [Table 12]. HAPH may also occur transiently in acclimatized lowlanders staying at HA and has been reported in lowlanders staying longer than 5–6 months at altitudes > 5000 m (16,000 ft) or following stay at EA for 6 months. The infrequent occurrence in most lowlanders may be due to their return to sea level once every 3–6 months while working at HA.
Etiopathogenesis: HAPH is believed to be the result of exaggerated and persistent hypoxic pulmonary vasoconstriction and remodeling of the pulmonary vasculature with muscularization of pulmonary arterioles leading to a chronically raised PAP.
Clinical features: Patients of HAPH are permanent residents or long-term sojourners at HA who present with dyspnea, cough, cyanosis, sleep disturbance, irritability, and features of right heart failure.
Diagnosis: A diagnosis of HAPH is considered in an individual presenting with the symptoms described above. The essential criteria to establish the diagnosis is raised pulmonary artery pressure (systolic PAP > 50 mmHg or mean PAP > 30 mmHg) measured at the altitude of residence and hemoglobin < 21 g/dL in males and < 19 g/dL in females [Table 12]. It is recommended that other causes of pulmonary hypertension, chronic obstructive pulmonary disease, interstitial lung disease, and other cardiovascular diseases associated with raised PAP should be ruled out before establishing the diagnosis of HAPH.
-
Treatment: Descent to lower altitude is the best therapy. For native highlanders for whom this may not be possible due to economic and social issues, the raised PAP can be reduced using calcium channel blockers (Nifedipine 20–30 mg 12-hourly), inhaled nitric oxide (NO) (15 ppm with 50% O2 or 40 ppm for 15 min), phosphodiesterase inhibitors, and prostaglandins.
Table 12: Diagnostic criteria and investigation findings in high altitude pulmonary hypertension (HAPH). A diagnosis of HAPH is established in an individual presenting with symptoms suggestive of HAPH who is found to satisfy both of the essential criteria given below.Essential criteria
Raised pulmonary artery pressure
Mean pulmonary artery pressure > 30 mm Hg and/or
Systolic pulmonary artery pressure > 50 mm Hg
Hemoglobin
Males < 21 g/dL
Females < 19 g/dL
Other findings
Chest X ray
Cardiomegaly – enlargement of right atrium and right ventricle
Prominent central and peripheral pulmonary arteries
ECG
Right axis deviation
Marked right ventricular hypertrophy
Echocardiography
Signs of right ventricular hypertrophy/failure
Complications and prognosis: Prompt descent to lower altitudes improves symptoms, and the PAP returns to normal in 12–16 weeks. Untreated, the condition results in worsening of right heart failure with patients developing severe shortness of breath.
Prevention: In view of a report on the occurrence of HAPH in a large number of soldiers posted for approximately 6 months at altitudes between 5800 and 6200 m (18,000–19,500 ft) and the large inter-individual variability in the response to hypoxia, it is recommended that lowlanders should not be stationed at EA for more than 3 months in an approximate 2-year duration.
B) Chronic mountain sickness (CMS) or Monge’s disease
Definition and epidemiology: First described by Carlos Monge in 1925, the consensus statement of the VI World Congress on mountain medicine 2004 defines CMS as a clinical syndrome that occurs in HA natives or long-term residents at altitudes above 2500 m, characterized by excessive erythrocytosis, severe hypoxemia, and in some cases moderate to severe pulmonary arterial hypertension, which may result in cor pulmonale, leading to congestive cardiac failure. Elevation of PAP and subsequent congestive heart failure are not mandatory features of CMS. The reported incidence of CMS in Tibetans is 0.91%.
Etiopathogenesis: CMS was attributed to a loss of acclimatization in already acclimatized individuals; however, a low hypoxic ventilatory response is no longer considered an important etiological factor. A likely possibility is markedly raised erythropoietin (EPO) levels. The raised hematocrit due to elevated EPO raises blood viscosity, worsens tissue hypoxia, and results in further elevation of EPO levels and leads to the genesis of a vicious cycle. Factors such as relative hypoventilation, sleep apnea, all hypopneas, overweight, and postmenopausal state are believed to be the important risk factors for developing CMS.
Clinical features and diagnosis: The clinical features of CMS are shown in Table 13. CMS is diagnosed clinically in patients living at altitudes greater than 2500 m, with normal lung function, and without chronic pulmonary or other medical conditions that worsen the hypoxemia. In patients with preexisting conditions that worsen hypoxia, the occurrence of features of CMS should lead to a diagnosis of secondary CMS.
-
Treatment: Descent to lower altitudes is the treatment of choice. Other modalities of treatment have variable results [Table 14].
Table 13: Clinical symptoms and signs of chronic mountain sicknessSymptoms
Headache
Breathlessness
Dizziness
Palpitations
Sleep disturbance
Fatigue
Localized cyanosis
Burning of palms and sole of feet
Dilatation of veins
Muscle and joint pains
Anorexia
Lack of mental concentration
Alterations in memory
Clinical/Investigative findings
Excessive erythrocytosis
Hemoglobin
(aa) > 19 g/dL in females
(ab) > 21 g/dL in males
Raised pulmonary arterial pressure (not mandatory)
Features of heart failure (not mandatory)
X-ray chest
Prominent pulmonary artery
ECG
Right ventricular hypertrophy
Right atrial enlargement
Proteinuria
ECG: Electrocardiogram
Table 14: Guidelines for management of chronic mountain sickness (CMS)Migration to lower altitudes is the treatment of choice
Other modalities of treatment with variable results
Phlebotomy to reduce hematocrit
Oxygen supplementation and respiratory training
Calcium channel blockers to reduce pulmonary artery pressure
Inhaled nitric oxide (40 ppm) or nitric oxide 15 ppm with 50% oxygen to lower pulmonary artery pressure
Medroxyprogesterone 20–60 mg/day for 10 weeks to stimulate ventilation
Tablet Acetazolamide 250 mg/day for 3 weeks
Complications and prognosis: CMS may result in cor pulmonale and congestive cardiac failure. Migration to lower altitude corrects the hypoxemia, and hematocrit returns to normal in 2–3 weeks. Right ventricular hypertrophy and elevated PAP if present normalize in about 2 years.
THROMBOSIS AT HIGH ALTITUDE
Both venous and arterial thrombotic events have been reported to have greater prevalence among lowlanders at HA and extreme HA.
Venous thrombosis: Isolated venous thrombosis (VT) at high altitudes has been frequently reported in the literature with various case reports of cerebral venous thrombosis (CVT), pulmonary thromboembolism (PTE), mesenteric vein thrombosis, and deep vein thrombosis (DVT) in apparently healthy individuals at HA. In the last one decade or so, a few case series based on the experience of the Indian Army in Ladakh have highlighted the issue of increased incidence of venous thrombosis at HA. An increase in the risk of venous thrombosis varying from 24.5 to 30 times higher in sojourners at HA compared to the plains has been suggested. A number of cases of thrombosis continue to be diagnosed regularly among troops at HA. Interestingly, a large number of these patients are young (<40 years) healthy individuals and thrombosis occurs within days to months after arrival at HA, at altitudes ranging from 3300–6000 m (11,000–20,000 ft), at unusual sites (cerebral, mesenteric, etc.), and in varied ethnic groups.
Mechanism and pathogenesis: Physical and environmental factors may cause a prothrombotic milieu at HA. Dehydration consequent to low ambient humidity, especially when an individual exerts and loses water by hyperventilation is a possible reason. Thirst mechanisms are known to be obtunded by ambient cold. The effect of increased procoagulant activity due to hypoxia and altitude is very significant. Immobility due to reduced daily activity, especially when bad weather supervenes and tight poorly fitting clothing and accessories, may further contribute to sluggish venous flow. Contrary to popular belief, anemia is a more important cause of thrombosis and not polycythemia. Thus, altitude coupled with environmental factors may precipitate a situation ideal for venous clot formation. This risk is compounded in patients predisposed to thrombosis by inherited thrombophilia such as Factor V Leiden mutation, protein C, protein S, and antithrombin deficiency. Platelet number and activation patterns may have a role to play as may endothelial dysfunction at HA. However, the contribution of these mechanisms, if existent at HA, has not been convincingly demonstrated yet.
Diagnosis and management: A high index of suspicion of thrombotic ailments is warranted to make an early diagnosis. The occurrence of HAPE/HACE in unusual settings, for example, late in the course of a stay at a given altitude, must arouse suspicion of PTE/CVT. Initiation of oxygen therapy and evacuation to lower altitudes are the two most important therapeutic steps at HA/EA. The patient must be evacuated to a secondary or tertiary care center at the earliest possible for further management. In the secondary or tertiary care center, the diagnosis must be confirmed by appropriate imaging modalities (Doppler for lower limb and abdominal veins, magnetic resonance venography (MRV) or contrast enhanced computed tomography (CECT) for cortical veins, and CT angiography for pulmonary vessels), and specific therapy instituted. All patients need to be started on anticoagulation. Heparin (low molecular weight or unfractionated heparin) may be started initially, followed by oral administration after an overlap of at least 5 days to keep a therapeutic INR of 2.5–3.0. Anticoagulation must be continued for at least 3 months for distal (calf vein) thrombosis and 6 months for proximal vein (popliteo-femoro-iliac or inferior vena cava (IVC) thrombosis. Long-term anticoagulation must be administered for thrombotic episodes such as PTE, CVT, and thrombosis at other unusual sites. Cases of pulmonary embolism may require thrombolysis depending on the presence of hemodynamic compromise and right ventricular dysfunction on echocardiography.
Arterial thrombosis: Arterial thrombosis is a recognized peril of high-altitude travel. Dehydration, hemoconcentration, cold, and prothrombotic milieu have been elucidated as etiologies for vascular thrombosis in high altitude, but evidence thus far is limited and also conflicting. Arterial thrombosis in high altitude has been reported in relation to cardiac, brain, mesenteric, and limb ischemia.
In patients with stable ischemic heart disease (IHD) at high altitude, signs of myocardial ischemia occur at similar or slightly reduced cardiac work, and left ventricular contractility is unaffected despite a possible reduction in coronary blood flow, suggesting that myocardial oxygenation is sufficient at least after a few days of acclimatization. Data at extreme altitude is, however lacking.4
Studies have shown that the incidence of cerebrovascular complications in young patients (<45 yr) is at least 10–12 times higher in high altitude. The risk of thrombotic events persisted for quite some time even after the individual left the HA area. Hence, it is advisory to follow the individuals up even after they have left HAA. Since there is a lack of sufficiently powered trials, the exact duration of follow-up cannot be dictated presently, but there is a definite need for a well-designed study to evaluate the mentioned queries.
HIGH ALTITUDE–ASSOCIATED SYSTEMIC HYPERTENSION (HASH)
Hypertension at high altitude
A number of soldiers, during fitness medical examinations after ascent to HA, are found to have blood pressure (BP) values in the hypertensive range, that is, >140/90 mmHg. The reasons for this may be
Increase in blood pressure upon ascent to HA and
Diagnosis of pre existing hypertension during medical examination at HA.
An increase in BP consequent to the stress of HA sojourn, such as hypobaric hypoxia, cold, and apprehension, may lead to BP values in the hypertensive range, especially in those with BP values in the prehypertensive range at near sea level. The elevation in BP seen at HA may be due to increased sympathetic discharge and fluid retention in the initial weeks at HA reported by some workers. The sympathetic discharge is believed to settle by 3 months of stay at HA. Few studies have examined BP over a 3-month period at HA, but emerging evidence from the ongoing longitudinal studies suggests a decrease in BP by 3 months of stay after an initial increase in the first 3–6 weeks’ stay. Data on the subsequent variations in BP is lacking.
In the absence of data on the amount and time course of BP elevation at HA, a management protocol is suggested [Figure 4] for subjects found to have hypertension during medical examination.
The effect of long-term (months to years) stay at HA on systemic BP has not been studied to date. Keeping in mind the likely effect of HA on endothelial function, the possibility exists that systemic hypertension may develop or be accelerated by HA exposure. Long-term longitudinal studies need to be conducted before this issue is set to rest.
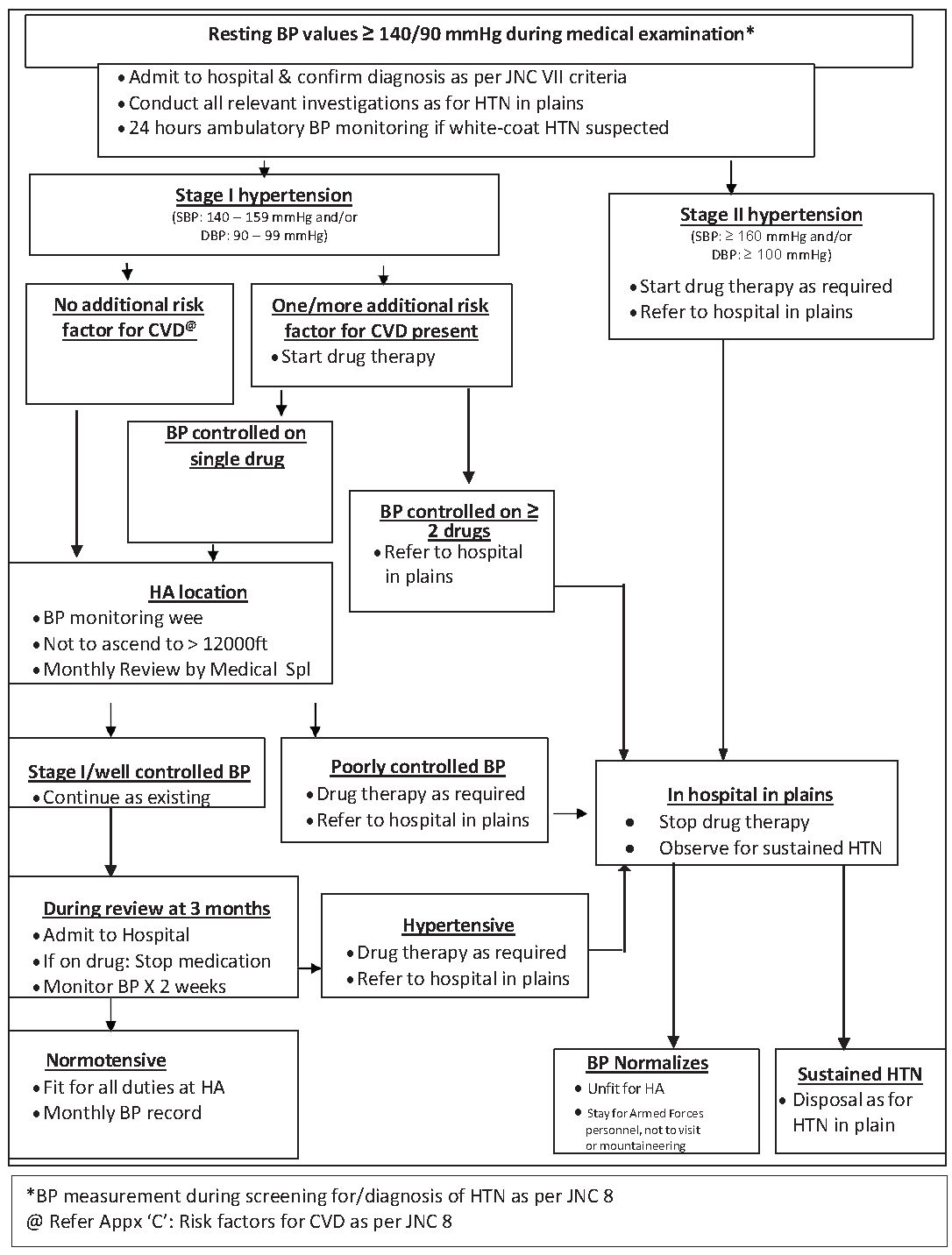
- Management protocol for subjects found to be hypertensive at HA. BP: Blood pressure, JNC: Joint national committee, HTN: Hypertension, CVD: Cardiovascular disease, HA: High altitude.
COLD INJURIES
Local cold injury: This includes chilblains, trench foot, and frostbite and usually affects peripheral structures such as fingers, toes, tip of nose, and ear lobes. The reason for this is the peripheral vasoconstriction that occurs on exposure to cold. Predisposing factors to local cold injuries are the wearing of tight clothing, contact with cold objects, smoking, immobility, old age, prolonged exposure to cold and moisture, chronic illness, fatigue, and past history of cold injury. Alcohol, by causing vasodilatation and by direct central effect on hypothalamus, can aggravate heat loss and thereby predispose to frostbite. Cold injuries are frequently associated with generalized hypothermia.
Chilblains: Also known as pernio, occur after prolonged exposure to non freezing temperatures and damp conditions. It is seen in susceptible individuals and consists of an aggravated or uncharacteristic response to cold exposure. The importance of chilblains lies in the fact that they are common, and so can affect the battle preparedness of soldiers. Patients with chilblains are prone to frostbite.
Pathophysiology: Prolonged exposure to cold results in constriction of the skin’s blood vessels, resulting in hypoxia. Edema in the dermis may also be present. Chilblain can occur with or without freezing of the tissue.7
Clinical presentation: The hands and feet are most commonly affected, but the chilblain of the thighs has also been reported. The affected part is red and there is intense irritation. Desquamation will be present, but tissue loss is rare. The lesions usually resolve spontaneously in 1–3 weeks, but they may recur in some individuals.
Treatment: Management consists of reassurance, keeping the part dry and warm, immersion in warm water followed by drying, and application of Vaseline and symptomatic treatment. Oral Nifedipine in a dose of 20–60 mg/day may be used to reduce the pain and speed up the resolution of the lesions.
Trench foot: Immersion foot or trench foot is observed in soldiers whose feet have been wet, but not freezing, for prolonged periods. It may occur at ambient temperatures near or slightly above freezing and is usually associated with dependency and immobilization of the lower extremities with constriction of the limb by shoes and clothing.
Clinical presentation: Immediate symptoms include numbness and tingling pain with itching, progressing to leg cramps and complete numbness. Initially, the skin is red; later, it becomes progressively pale and mottled and then gray and blue. The soles of the feet are wrinkled and very tender to palpation. The progression of this cold injury has three stages.
Stage 1: Prehyperemic phase, lasting for a few hours to a few days, in which the limb is cold, slightly swollen, discolored, and possibly numb. Major peripheral pulses are barely palpable.
Stage 2: The hyperemic phase lasts 2–6 weeks. It is characterized by bounding, pulsatile circulation in a red, swollen foot.
Stage 3: Post-hyperemic phase lasts for weeks or months. The limb may be warm, with increased sensitivity to cold. The injury often produces superficial, moist, liquefaction gangrene quite dissimilar to the dry, mummification gangrene that occurs with severe frostbite.
Treatment: Management of this injury entails careful washing and air-drying of the feet, gentle rewarming, bed rest, and slight elevation of the extremity. Improvement occurs within 24–48 hours, while the injury completely resolves in 1–2 weeks.
Frostbite: This is the most serious of local cold injuries and is usually seen at temperatures below freezing point. The pathophysiological process of frostbite may be divided into four phases: prefreeze, freeze–thaw, vascular stasis, and late ischemic. Overlap is usually present between the phases. In the early prefreeze phase, vasoconstriction and ischemia occur with neuronal cooling and ischemia leading to pain and paresthesia. Subsequently, there is freezing of intracellular or extracellular fluid with the formation of ice crystals, leading to the rise in osmotic pressure, intracellular dehydration, electrolyte imbalances, protein and lipid derangement, cell membrane injury, and cell death. A reversal of this process probably occurs during the thawing of frozen tissues. After tissue thawing, vasodilation and leakage from capillaries occur, causing tissue edema. Alternating freeze thaw cycles potentiate vascular injury lead to ischemic death of tissues. The vasoconstriction and stasis seen in frostbite are associated with the release of prostaglandins that have been implicated in progressive dermal ischemia. Both prostaglandin F2 and thromboxane A2 cause platelet aggregation and vasoconstriction. Therapy with antiprostaglandin agents and thromboxane inhibitors has been shown to increase tissue survival. Frostbite develops as a function of the body’s protective mechanisms to maintain core temperature. Warm blood is shunted from cold peripheral tissues to the core by vasoconstriction of arterioles of the extremities and face, especially the nose and ears. Hypothermia may be seen along with frostbite. Frostbite progresses from distal to proximal and from superficial to deep. The severity of frostbite is proportional to the duration of exposure to cold.8
Clinical features: The onset is usually insidious, with pain and numbness followed by loss of sensation of the affected part. Initially, as the tissue is freezing, the patient experiences discomfort or pain. This progresses to numbness and loss of sensation. Upon examination, the frozen tissue is white and anesthetic, owing to intense vasoconstriction. Tissues that remain frozen can appear mottled, violaceous, pale yellow, or waxy. Favorable signs include warmth, normal color, and some sensation. It has been demonstrated repeatedly that a person who previously suffered frostbite is more prone to develop this cold injury in the same body part than an individual with no history of such a cold injury.
Classification of frostbite: In very early stages, there is the freezing of the most superficial layers of epidermis, producing a blanched wheal, which is called frostnip. Frostnip, the mildest form of cold injury to the skin, is a precursor to frostbite. It can also occur from skin contact with cold surfaces (e.g., metal, equipment, liquid). Mild frostbite involves freezing of the skin and adjacent subcutaneous tissues; extracellular water freezes first, followed by cell freezing. Severe frostbite is the freezing of the tissues below the skin and the adjacent tissues, which can include muscle, tendon, and bone. Traditionally, frostbite has been classified into four degrees of severity, as follows:
In first-degree frostbite, hyperemia and edema are evident.
Second-degree frostbite is characterized by hyperemia and edema, with large, clear blisters that may extend to the entire length of the limb, digit, or facial feature.
Third-degree frostbite is characterized by hyperemia, edema, and vesicles filled with hemorrhagic fluid that are usually smaller than those of second-degree frostbite and do not extend to the tip of the involved digit.
Fourth-degree frostbite, the most severe type, involves complete necrosis with gangrene and loss of the affected part.
A simpler and clinically more relevant classification divides frostbite injury into two types: superficial or deep.
Superficial frostbite: (First and second degree frostbite): Involve the skin and subcutaneous tissues. The skin is cold, waxy white, and non-blanching. The frozen part is anesthetic, but becomes painful and flushed with thawing. Edema develops and clear bullae filled with serous fluid appear within the first 24 hours.
Deep frostbite: (Third- and fourth-degree frostbite): Involves the muscle, tendons, neurovascular structures, and bone, in addition to the skin and subcutaneous tissues. The frozen part is hard, wood like, and anesthetic. It appears ashen-gray, cyanotic, or mottled and may remain unchanged even after rewarming. Edema develops, but bullae may be absent or delayed. Bullae, if present, are filled with hemorrhagic fluid.
Treatment in peripheral medical facilities or prehospital care: The patient must be removed from the cold environment. At level of the most peripheral medical facility, general warmth should be provided by hot fluids, sleeping bags, and extra blankets and associated hypothermia, if present, should be dealt with. The patient should be reassured, given mild analgesics, dry dressing applied to affected parts, tetanus toxoid given, and ibuprofen should be started in a dose of 12 mg/kg per day in two divided doses (minimum to inhibit harmful prostaglandins) to a maximum of 2400 mg/day in four divided doses. Aspirin has been proposed as an alternative for anti-inflammatory and platelet inhibition effects and may be given if ibuprofen is not available. Vasodilators such as Pentoxifyline, Nifedipine, and Phenoxybenzamine have been used as primary or adjunctive therapies in the treatment of frostbite. Pentoxifylline, if available, should be given in a dose of 400 mg thrice daily. The patient should be transferred to the nearest hospital at the earliest. Treatment should not be attempted in the field if a hospital is available within a short distance or if a risk exists that the extremity will be refrozen during transportation. Once the rewarming process has begun, weight-bearing on the affected part is almost certain to result in additional injury. Rubbing the frostbitten part with snow or exercising it in an attempt to hasten rewarming is absolutely contraindicated. Contrary to popular belief, walking some distance on frostbitten feet can result in aggravation of damage. Consequently, this should be avoided.
Treatment in a hospital setup: Normal body temperature should be restored before treating the local injury. The preferred initial treatment for frostbite is rapid rewarming in a water bath at a temperature of 39–42°C (102.2–107.6°F). Strict aseptic technique (e.g., mask, powder-free gloves) should be used by all personnel during the warming procedure and during subsequent wound treatments. The rewarming bath should be large enough so that the frostbitten part does not rapidly reduce the temperature of the water. The temperature of the bath should be monitored carefully as the bath cools. Additional hot water may be added to the bath only after the extremity is removed from it. After the addition of hot water, the bath should be stirred, and the temperature retested before the extremity is reintroduced into the bath. Rewarming should be continued until the frostbitten tissue has a flushed appearance, demonstrating that circulation is reestablished. This rewarming procedure usually lasts 30–45 minutes. Since rewarming is painful, narcotics are often required. After rewarming, the skin should be washed gently and then carefully dried.
Once rewarming has begun, it is imperative that the affected tissue not be allowed to refreeze, as this may result in tissue necrosis. The use of heparin, low molecular weight dextran, and oral anticoagulants have shown no beneficial effects, and their use is not advocated. The benefit of prophylactic antibiotics continues to be debated, and their use is reserved for specific infectious complications. A therapeutic approach should be devised to prevent the progressive dermal ischemia of frostbite. Systemic ibuprofen may be used to reduce dermal ischemia. The goal of thrombolytic therapy in frostbite injury is to address microvascular thrombosis. For deep frostbite injury with potential significant morbidity, use of intravenous or intra-arterial tissue plasminogen activator (tPA) in the dose of 0.15 mg/kg bolus followed by 0.15 mg/kg/hour infusion over 6 hours (to a maximum of 100 mg) and heparin in a dose of 500 mg/hour for 3–5 days within 24 hours of thawing may salvage some or all tissue at risk in Class 4 frostbites without any trauma or bleeding. For those with Class 3 frostbite trauma or those beyond the window period of up to 72 hours, Iloprost (prostacyclin analog) is indicated. The tissue plasminogen activator is continued till there is evidence of tissue reperfusion or for a maximum of 48 hours. Common indications for use of thrombolytic therapy are purple discoloration of the affected part, absent capillary refill, or the presence of hemorrhagic blisters. The clear blisters are debrided immediately, and antiseptic cream is applied directly to the debrided wound. In contrast, the hemorrhagic blisters are left intact. When the hemorrhagic blisters rupture, these should be debrided. Topical aloe vera cream or gel should be applied to the thawed tissue prior to applying dressings. Aloe vera cream or gel can be reapplied at each dressing change, or every 6 hours. If nonsteroidal anti-inflammatory drugs (NSAIDs) have not been initiated in the periphery, ibuprofen should be administered at a dose of 12 mg/kg divided twice daily, unless contraindicated (e.g., history of allergy, peptic ulcer disease) until the frostbite wound is healed or surgical management is undertaken (typically 4–6 weeks). The affected part should be protected from trauma and infection, and it should be elevated above the patient’s heart to minimize edema. A protective cradle should cover frostbitten lower extremities to prevent trauma. An environmental temperature of 21–26°C (69.8–78.8°F) in the hospital room is usually comfortable for the patient. Patients with first- or second-degree frostbite of the feet should continue bed rest until the edema has receded and the vesicles and bullae have dried, which usually takes 2 weeks. Patients with more severe frostbite should remain in bed until wound repair is complete. Avoidance of joint stiffness and wound contraction is an essential goal of the rehabilitation program.
As a general rule, amputation and surgical debridement should be delayed for 60–90 days unless severe infection with sepsis develops. The natural history of most injuries is one of gradual demarcation of the injured area, followed by dry gangrene or mummification of the area, with later sloughing of necrotic tissue, resulting in a viable but shortened extremity beneath the eschar. Emergency surgery is occasionally required for patients with a frostbitten extremity. Open amputations are indicated in patients with persistent infection with sepsis that is refractory to debridement and antibiotics. Compartment syndrome may be encountered in a frostbitten extremity, which mandates fasciotomy.
Prevention: Prevention is the most important strategy in the management of frostbite and is aimed at ensuring adequate perfusion of tissues and prevention from cold [Table 15].
|
|
|
HYPOTHERMIA
Body temperature regulation depends on a balance of heat generation and loss. In the extreme cold conditions seen in the winters at HA and round the year in EA, body heat loss in an inadequately dressed individual could rapidly lead to hypothermia. Since clothing is mostly of is of appropriate quality, hypothermia rarely if ever, occurs today. However, we should be alert to the possibility at all times, especially in survivors of avalanche accidents, blizzards, and falls into lakes or rivers and crevices in ice. Injured or ill persons have an increased risk of cooling, and unusually rapid cooling should arouse suspicion of underlying injury or illness. A reduction of core body temperature below 35°C is known as hypothermia.
The occurrence of hypothermia in an otherwise healthy person is known as primary hypothermia and is the result of greater heat loss than production. This is more likely when the metabolic energy substrate stores are depleted. Secondary hypothermia may occur in ill persons with a variety of underlying ailments, even in warm environments due to impaired thermoregulation and/or increased heat loss. CVA, central nervous system (CNS) injury, subarachnoid hemorrhage, acidosis, fatigue, hypoglycemia, and certain toxins may impair thermoregulation; burns, cold infusions, hyperdynamic circulation states, infections, multisystem trauma, and shock may increase heat loss, predisposing to hypothermia.
Diagnosis: A diagnosis of hypothermia should be considered in the setting of a history of cold exposure or a disease that predisposes to hypothermia, for example, head injury and the finding of a cold body or trunk in a patient. The core body temperature measuring a value of <35°C confirms the diagnosis of hypothermia. A rectal thermometer inserted to a depth of 15 cm/esophageal probe in the lower third is required to record the core body temperature in hypothermic patients. Traditionally, hypothermia has been classified as mild, moderate, severe, and profound. However, in a peripheral setting, where core body temperature cannot be measured, the Swiss Staging system that depends on clinical signs is preferable, especially since it aids decision-making on the management and transportation of the patient. Five stages of hypothermia are recognized based on the level of consciousness, shivering, and presence of vital signs [Table 15]. Absence of vital signs, an incompressible chest, and stomach muscles that cannot be kneaded (whole body frozen solid) are features of death due to irreversible hypothermia. Rigor mortis and fixed dilated pupils may be present in patients of reversible hypothermia.
Clinical features: Consciousness, breathing, and circulation are initially intact in all types of hypothermia but are impaired as the body cools further. The body responds initially to cold by shivering and an increase in voluntary movement. At core temperatures <28°C (82°F), some patients may engage in paradoxical undressing. At core temperatures <32°C, atrial fibrillation is common but benign in the absence of other features of cardiac instability. Systolic BP <90 mmHg, ventricular arrhythmias, and core temperature <28°C suggest or cause cardiac instability. The risk of cardiac arrest also increases below 32°C and is significantly greater below 28°C. The patient may be disoriented, confused, or drowsy and slip into a coma with greater hypothermia. On physical examination, there is pallor, the skin is ice cold, and the respiration, heart rate, and blood pressure may be increased early in hypothermia but later decrease and may be difficult to measure. This may lead the clinician to believe that the patient is dead. However, severely hypothermic patients with cardiac asystole can be resuscitated successfully even after a few hours of cardiac arrest. The immediate danger to life is from ventricular fibrillation, which is precipitated by any muscular activity, and the patient must be handled with minimal manipulation. Terminally, pulmonary edema supervenes.
Management: A suggested scheme for out-of-hospital management of hypothermia is as shown in Figure 5.9
Effective and continuous cardiopulmonary resuscitation (CPR) is the mainstay of therapy for patients of stage IV hypertension. Survival up to 390 minutes after CPR has been documented in cases of HT IV. Airway management and CPR must be initiated, and the patient must be transported without delay to a hospital. CPR must be continued till the body is rewarmed to 32°C and cardiac stability is achieved. Indications for termination of CPR include:
Obvious features of death, such as decapitation, whole body frozen solid, and drowning (in water or snow)
-
Absence of vital signs with body temperature >32°C and/or serum potassium level >12 mmol/L.
Patients with a history or features suggestive of cardiac arrest before cooling have a poorer outcome on resuscitation. Organ failure is common 24 hours after admission, and pulmonary edema is the commonest cause of death.
Rewarming techniques: A warm environment, warm clothing, and ingestion of hot, sweet drinks where possible, are required for all patients of HT. Rewarming techniques include:
Active external and
-
Invasive rewarming
The latter may be minimally invasive (warm parenteral fluids) or include methods such as peritoneal lavage, thoracic lavage, hemodialysis, veno-venous or veno-arterio extracorporeal membrane oxygenation (ECMO), and cardiopulmonary bypass (CPB). Invasive rewarming techniques have attendant risks, such as bleeding, and are suggested only for cases with cardiac instability or HT IV (other than IV fluids, which may be administered in HT II and III with cardiac stability). Where ECMO and CPB are not available, thoracic lavage may be preferred. Active external rewarming employs the use of chemical packs, electric blankets, and forced-air blankets. The risk of ventricular fibrillation and cardiac arrest is ever present when rewarming a case of hypothermia, and the Medical Officer (MO) or physicians should be alert of this possibility. Cardiac resuscitation should be available when rewarming.

- Suggested algorithm for the management of hypothermia in peripheral setting (based on WMS consensus guidelines 2014 as updated). CPR: Cardiopulmonary resuscitation, ECC: Emergency cardiovascular care, WMS: Wilderness medical society, IV: Intravenous, IO: Intraosseous, ICU: Intensive care unit, ECG: Electrocardiogram.
CONCLUSION
High-altitude illnesses are a major detriment to living and working in high altitudes for lowlanders. The features of AMS include headache, loss of appetite, sleep disturbance, nausea, fatigue, and dizziness, which begin shortly after rapid ascent to high altitude. The biggest issue in this case is that due to the ease of availability of air travel, rapid ascent has become a rule rather than an exception, which has exponentially increased this condition in the recent past. Hypoxia in HA can lead to impaired mental performance, weight loss, and reduced capacity for exercise. Certain factors, such as elevation from where a person comes to HA, maximum altitude reached, sleeping altitude (it is best to work high and sleep low), rate of ascent, latitude, intensity of exercise, pre-acclimatization, prior high-altitude experience, and genetics impact an individual’s propensity for AMS. The symptoms are relieved by rest and withholding further ascent till symptoms are resolved. In severe cases, descent to a lower altitude is always curative. AMS may progress to HACE. HAPE can intervene without AMS. These two conditions, unlike AMS, are medical emergencies and need descent, supplemental oxygen, and in the case of HACE, the use of oral steroids. Nifedipine, and more recently phosphodiesterase-5 inhibitors, may be effective in the management of HAPE. Descent should never be alone. Portable hyperbaric chambers must be considered if descent is not possible. A high index of suspicion is needed to diagnose these acute life-threatening disorders at high altitudes. Awareness of subacute and chronic high-altitude illnesses as well as miscellaneous disorders of high altitudes, including cold injuries as well as other conditions aggravated by high altitude among medical professionals would go a long way in their prevention, early detection, and treatment.
Acknowledgment
We would like to acknowledge Maj Gen Manas Chatterjee, Col Arun Kumar Yadav, Lt Col Dharmendra Kumar for their contribution.
References
- High-altitude illness. Emerg Med Clin North Am. 2004;22:329-55.
- [CrossRef] [PubMed] [Google Scholar]
- Acute mountain sickness, high altitude cerebral oedema, high altitude pulmonary oedema: The current concepts. Med J Armed Forces India. 2008;64:149-53.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- High-altitude illnesses: Old stories and new insights into the pathophysiology, treatment and prevention. Sports Med Health Sci. 2021;3:59-69.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Can patients with coronary heart disease go to high altitude? High Alt Med Biol. 2010;11:1024.
- [Google Scholar]
- Environment-dependent sports emergencies. Med Clin North Am. 1994;78:305-25.
- [CrossRef] [PubMed] [Google Scholar]
- High-altitude pulmonary edema. In: Hornbein TF, Schoene RB, eds. High Altitude: An exploration of human adaptation. New York: Marcel Dekker; 2001. p. :782.
- [Google Scholar]
- National athletic trainers’ association position statement: Environmental cold injuries. J Athl Train. 2008;43:640-58.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Wilderness medical society clinical practice guidelines for the out-of-hospital evaluation and treatment of accidental hypothermia: 2019 update. Wilderness Environ Med. 2019;30:S47-69.
- [CrossRef] [PubMed] [Google Scholar]




