Translate this page into:
Tuberculous pericarditis: Molecular methods to aid early diagnosis and treatment to avoid sinister outcomes
* Corresponding author: Dr. Urvashi Singh, All India Institute of Medical Sciences, New Delhi, India. drurvashi@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Roy P, Bala K, Biswas J, Ahmed J, Bir R, Yadav VS, et al. Tuberculous pericarditis: Molecular methods to aid early diagnosis and treatment to avoid sinister outcomes. Ann Natl Acad Med Sci (India). doi: 10.25259/ANAMS_151_2024
Abstract
Objectives
Tuberculous pericarditis (TP) can have a varied clinical picture and is a differential diagnosis in non-self-limiting pericarditis. Diagnosing TP early is challenging due to the low concentration of mycobacteria in pericardial fluid. An early diagnosis can expedite treatment and response. The present paper summarises laboratory experience with the diagnosis of TP.
Material and Methods
The study included patients with a clinical suspicion of TP (2018-2022). Pericardial fluid samples were analyzed using microscopy (Ziehl Neelsen staining or ZN), GeneXpert MTB/RIF (Gx), TB PCR, and liquid culture (mycobacteria growth indicator tube [MGIT 960]). Not all samples could be tested using every method. The NALC-NaOH method was used for sample processing. Blood samples of the patients were tested for lymphocyte count and erythrocyte sedimentation rate (ESR). The samples were also tested for adenosine deaminase (ADA) using a commercial kit. Statistical analysis was performed using Stata 16.0 software, with p-values calculated using the Wilcoxon rank-sum test.
Results
In all, results from 230 patients were included for analysis. Six patients detected positive for TB, with a mean age of 48 years, and 4 patients were male. None of the samples tested positive by ZN staining, 5/6 samples were positive by molecular techniques (2 by Gx and 3 by PCR), and the culture was positive by MGIT in one person. Neither serum ADA enzyme values nor ESR were significantly associated with TP. Of six positive patients, two had normal lymphocyte counts, while the rest of the four patients had a low lymphocyte count. Sixteen patients had high lymphocyte counts but were not positive for TB. Two of the six TB-positive patients succumbed to the disease; one of these two patients was suffering from cancer as well. Two were lost to follow-up, while 2 others recovered.
Conclusion
Molecular methods contributed to early diagnosis. Lymphocyte count, serum ADA levels, and ESR were not found to be significantly associated with TP. Hence, we advocate the use of molecular methods for early diagnosis of TP, which will also reduce sinister outcomes like mortality.
Keywords
Adenosine deaminase
GeneXpert
MGIT 960
TB PCR
Ziehl Neelsen stain
INTRODUCTION
Tuberculous pericarditis (TP) presents a varied clinical picture and should be considered in the evaluation of cases of pericarditis that do not resolve on their own. Although the overall incidence of tuberculosis (TB) has decreased, the rate of extrapulmonary TB remains as is1 with around 1,050,000 new cases reported globally in 2018.2,3 In developing countries, TP occurs in about 1-2% of patients with pulmonary TB, but it can also appear as an isolated extrapulmonary manifestation.4
TP usually progresses gradually, presenting with nonspecific systemic symptoms such as night sweats, fever, weight loss, and fatigue. While chest pain, cough, and dyspnea are common, the sharp, acute chest pain typical of idiopathic pericarditis is uncommon in TP.5
The challenges in diagnosing TP lie in confirming a tuberculous cause.4 Despite thorough investigation, 15–20% of pericardial disease cases remain undiagnosed.6 Early diagnosis is complicated by the low number of mycobacteria present in the pericardial fluid, leading to a low yield in acid-fast mycobacteria smears.7 Culture-based confirmation of mycobacteria is time-consuming and not practical for early diagnosis.8 However, advances in molecular techniques, which amplify specific genetic material from M. tuberculosis, have significantly improved diagnostic speed.7 Additionally, detecting rifampicin resistance, indicated by mutations in the rpoB gene, can be particularly useful in regions with high rates of multidrug-resistant TB.2
The present paper summarizes laboratory experience with the diagnosis of TP.
MATERIAL AND METHODS
In this study, we analyzed pericardial fluid samples collected between January 1, 2018, and December 31, 2022. These samples were tested using Ziehl–Neelsen (ZN) staining, GeneXpert MTB/RIF (Gx), TB PCR, and liquid culture (Mycobacteria growth indicator tube [MGIT] 960). The ZN smear test was conducted for all patients. All the samples could not be subjected to GeneXpert, tuberculosis-polymerase chain reaction (TB-PCR), and liquid culture. The processing of samples involved the NALC-NaOH method, which includes treating clinical samples with n-acetyl L-cysteine (NALC), 4% sodium hydroxide, and sodium citrate for 15 minutes, followed by neutralization with phosphate-buffered saline (PBS). The samples were then centrifuged at 3000 rpm for 30 minutes. Following this, the samples underwent a series of four tests: microscopy (using ZN staining), GeneXpert MTB/RIF (Gx), TB PCR, and liquid culture (MGIT 960).9
Decontaminated samples were stained using the ZN technique, and results were reported according to the National Tuberculosis Elimination Program guidelines.10
After sample processing, concentrated sediment was inoculated into an MGIT 960 medium and incubated at 37°C. It is a liquid medium for mycobacterial culture. The MGIT 960 system utilizes a fluorescent compound sensitive to dissolved oxygen. Initially, the fluorescence decreases due to high oxygen levels, but as microorganisms consume oxygen, fluorescence increases. This change is monitored to identify viable organisms. Tubes that do not show growth after 42 to 56 days are deemed negative and removed from the system.
For the Xpert MTB/RIF assay, which utilizes heminested real-time PCR, testing was conducted following the manufacturer’s guidelines. This assay can detect both the Mycobacterium tuberculosis complex (MTBC) and rifampin resistance in less than two hours. Additionally, TB PCR was performed to detect the MPT 64 gene. DNA was extracted via heat lysis followed by chloroform extraction and then amplified using gene-specific primers, Taq polymerase, and dNTPs.
Blood samples of the patients were tested for lymphocyte count and erythrocyte sedimentation rate (ESR) as per the standard protocol.11
The samples were tested for adenosine deaminase (ADA) using a commercial kit from Tulip Diagnostics Private Limited.
Statistical analysis was performed using the Stata 16.0 software, with p-values calculated by the Wilcoxon rank-sum test (Mann-Whitney test). Statistical significance was set at a p-value of less than 0.05.
RESULT
Total 230 samples were reported between 1st Jan 2018 and 31st December 2022. Year-wise sample distribution is shown in Table 1.
| Year | Frequency |
|---|---|
| 2018 | 36 |
| 2019 | 35 |
| 2020 | 36 |
| 2021 | 64 |
| 2022 | 59 |
| Total | 230 |
Samples were evaluated using ZN staining, GeneXpert, PCR, and MGIT. Figure 1 illustrates the distribution of samples and the positive results obtained by each method. ZN staining did not yield any positive results. GeneXpert identified two positive samples, while three samples tested positive by PCR. Additionally, one patient’s culture was positive via MGIT.
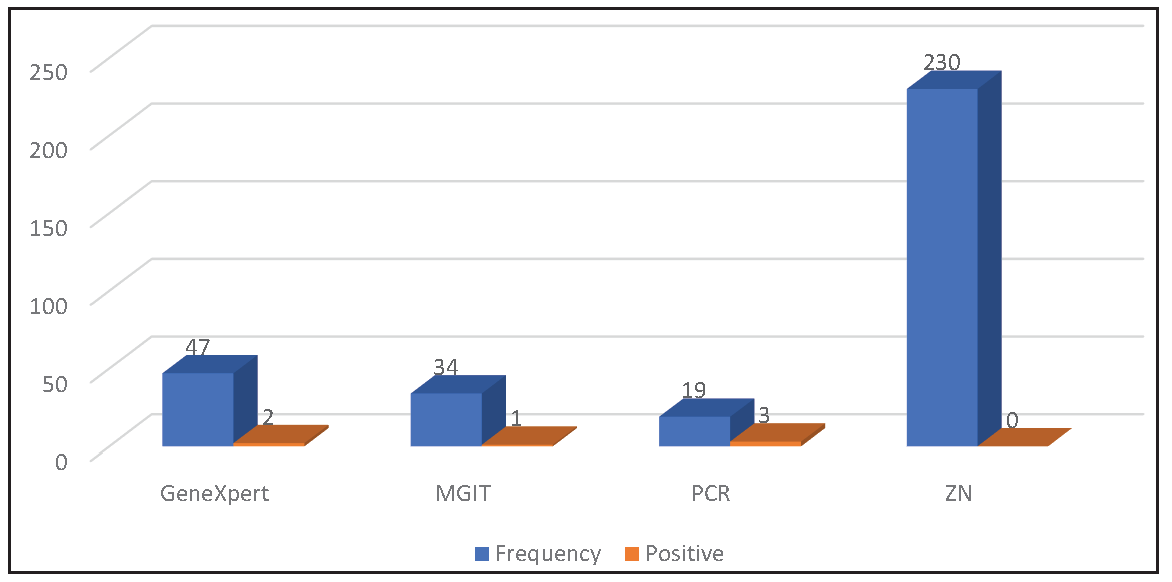
- Test-wise sample distribution and positive result obtained by the techniques. ZN: Ziehl–Neelsen, PCR: Polymerase chain reaction, MGIT: Mycobacteria growth indicator tube.
The ratio of male to female patients was 2:1 (p-value: 0.664). In total, six patients tested positive, resulting in a prevalence rate of 2.6%. The average age of the positive patients was 48 years.
Table 2 presents the lymphocyte percentage, ESR, and ADA levels for the patients.
| Parameter | Result for TB | p- value | |
|---|---|---|---|
| + | - | ||
| Median (Q1,Q3) | Median (Q1,Q3) | ||
| Lymphocyte % | 17.3 (10.4, 35.6) | 17.4 (10.3, 28.9) | 0.814 |
| ESR | 26 (8, 66) | 35 (20, 51) | 0.7176 |
| ADA | 10 (10, 10) | 26 (18, 35) | 0.1576 |
Q: Quartile
Of six positive patients, two had a normal lymphocyte count, while the rest of the four patients had a low lymphocyte count. Sixteen patients had a high lymphocyte count but were not positive for TB.
ESR (p-value – 0.7176) and ADA (p-value- 0.1576) were not significantly associated with TB.
Two of the six patients succumbed to the disease; one of them suffered from cancer as well. Both the patients who succumbed to the disease were positive by molecular tests. Two were lost to follow-up, while two others recovered.
Clinical vignette
-
1.
A 46-year-old woman, resident of Kotla, Delhi, presented in 2018. An ultrasound abdomen revealed a large, well-defined cyst in the pelvis, mainly on the right side. On MRI, the pelvis also showed a large right paramedian right adnexal cystic lesion. While undergoing evaluation for the right ovarian cyst, the patient was found to be suffering from tubercular pericardial effusion as well [Figure 2]. Genexpert was positive. Ascitic fluid GeneXpert was also positive for M. tuberculosis, sensitive to rifampicin. Laboratory tests showed lymphocytes to be 35.6%. According to the data presented, a diagnosis of tuberculous pericarditis was confirmed, with the strain identified as sensitive to rifampicin. She was treated for TB and recovered.
-
2.
A 75-year-old woman, resident of Orissa, presented in 2018 for investigation and treatment of an inguinal hernia. Abdominal ultrasound was performed, in which pericardial effusion was also detected incidentally. She was further investigated for TP. MGIT was positive. She was treated for TB and recovered.
-
3.
A 33-year-old male, resident of Uttar Pradesh, presented in 2021, and was being treated for both cancer and TB. TB was detected by TB-PCR. However, the cancer had metastasized, and after a few months he ultimately succumbed to the disease.
-
4.
A 19-year-old male, resident of Haryana, presented in 2021. He underwent a surgical procedure for endocarditis at our hospital. A week after the surgery, his GeneXpert report was positive, resistant to rifampicin. He also underwent TB treatment for 1 week, after which he expired.
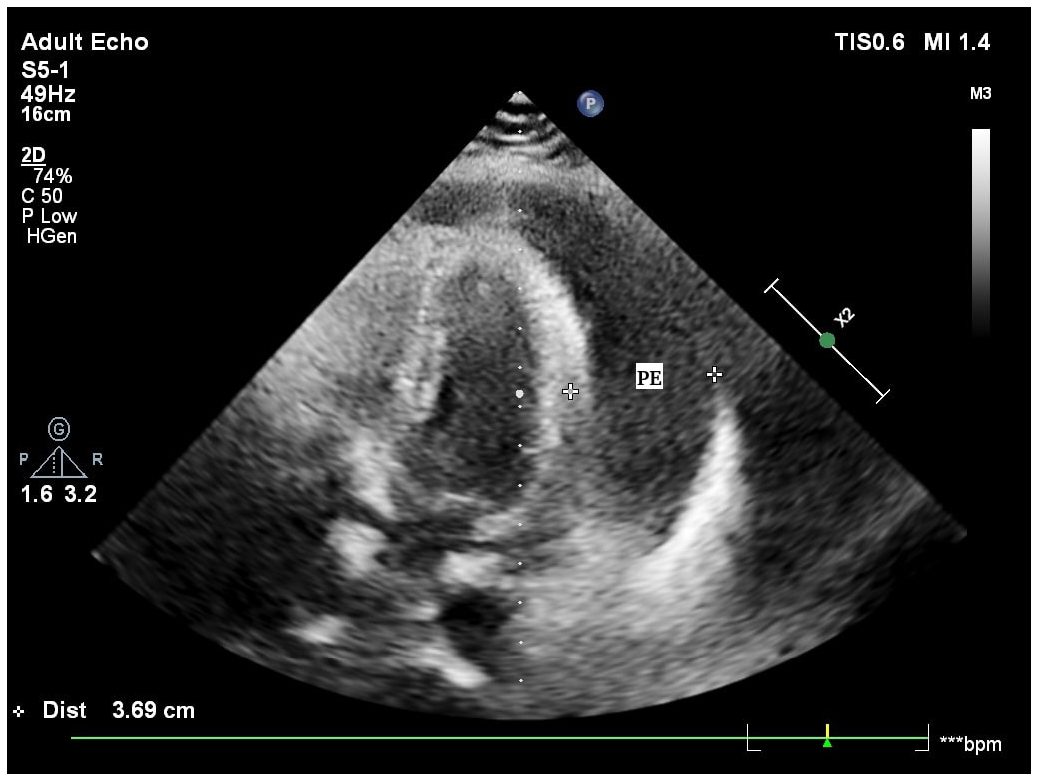
- Echocardiogram of a patient diagnosed to have tuberculous pericardial effusion (PE).
DISCUSSION
In developing countries, TB is a leading cause of pericarditis. In contrast, TB in developed nations accounts for fewer than 5% of pericarditis cases, with viral infections being responsible for 80% to 90% of cases.12 TP typically represents about 4% of acute pericarditis cases, 7% of cardiac tamponade cases, and 6% of constrictive pericarditis cases.13 However, in the current study, the prevalence of TP was found to be 2.6%. This could be due to the study period, spanning 1st January 2018 to 31st December 2022, overlapping with the COVID-19 pandemic. The majority of the samples were from the post-covid period (54%). The pre-covid period accounted for 16% of the samples, and the remaining 30% samples belonged to the post-covid period. Maximum concentration of the positive cases (67%) was found in the pre- and post-covid periods.
TP is more commonly associated with males, as compared to females.14,15 A similar finding has been reported in our study, with the male:female ratio being 2:1. The reasons behind sex differences in pericardial inflammation remain unclear. However, experimental research on myocardial inflammation indicates that sex hormones may significantly influence this disparity.15,16 Testosterone, in particular, seems to be a major factor in the development of myocarditis. Studies have shown that administering exogenous testosterone exacerbates heart inflammation, while gonadectomy reduces cardiac inflammation in experimental models of myocarditis.15,17 Testosterone’s effects are thought to involve the suppression of anti-inflammatory cells and the promotion of a Th1-type immune response.15,18
The age group involved in TB has comprised young patients in most studies.19 Our findings align with this trend, with a mean age of 48 years among patients diagnosed with TP.
Diagnosing TP remains challenging due to the lack of a straightforward, rapid, and widely available test, despite the condition’s significant morbidity and mortality. Typically, pericardial fluid in TP cases is a protein-rich lymphocytic exudate, often with gross hemorrhage.2
The confirmation of TP diagnosis can be achieved through one of the following criteria: a positive culture for M. tuberculosis in the pericardial fluid, a positive direct examination for M. tuberculosis, or an ADA level greater than 50 IU/L.20 Additionally, a pericardial biopsy can confirm TP if it reveals a positive culture for M. tuberculosis, granulomas with caseous necrosis, langhans-type multinucleated giant cells, or the presence of tubercle bacilli.21
Pericardial fluid culture remains the most commonly used diagnostic method for TP, with a sensitivity of 53% to 75%. However, results from this test typically take at least three weeks to be obtained.2,22,23
Methods based on nucleic acid detection have significantly improved pathogen disease diagnosis. A molecular diagnostic technique yields results much faster than the classical methods, which allows timely management of the disease and thus decreases the possibility of multi-drug resistance of TB. The PCR test for detecting Mycobacterium tuberculosis nucleic acid in pericardial fluid is generally more accessible and cost-effective compared to PCR on pericardial tissue. However, it has lower sensitivity (15% versus 80%) and can produce up to 20% false positives.24 Despite these limitations, PCR remains a valuable tool due to its ability to detect various nucleic acid sequences, even in samples with low bacilli counts. Additionally, PCR can identify rifampicin resistance through mutations in the rpoB gene, which is particularly useful in areas with high rates of multidrug-resistant TB.2
Diagnostic yield of Gx in pericardial fluids is reported in the range of 40%-100% and specificity in the range of 72-100%.25 Collection of pericardial fluid samples which are free of blood (blood can contaminate sample during collection) can be crucial for the performance of any PCR. Known inhibitors of PCR in blood includes hemoglobin and immunoglobulin G. Hemoglobin affects DNA polymerase activity and is known to quench free dye fluorescence, while immunoglobulin binds to the genomic DNA, affecting DNA polymerization.26 Addition of anticoagulants such as heparin, to fluids, such as peritoneal or pleural fluid after collection, could aid in improving the yield of PCR, as clots and enzymes activated during the clotting process can also lead to PCR inhibition.27
No new technologies or methods have emerged to improve the low yield of pericardial fluid smear tests for acid-fast bacilli (AFB). Despite this, the high specificity of positive microscopy may justify its continued use.28 Given that TP fluid is paucibacillary, the diagnostic accuracy of smear tests is estimated to be only 5%.25 This explains the study’s findings: although AFB staining was performed on more than three times the number of samples compared to molecular techniques (100% vs. 28.5% for PCR and GeneXpert, and 14.5% for MGIT), none of the samples tested positive via ZN staining. In contrast, 7.6% of samples were positive by molecular techniques, and 2.9% were positive by MGIT.
Lymphocytes are critical in the immune response to mycobacterial infections.29 Hence, they are often raised in TB patients. However, in the present study, most of the TP patients (67%) had low lymphocyte levels, while the rest (33%) had normal lymphocyte levels. Similar observations have been reported by Deepinder Chhina et al. (2013) and H. Reuter et al. 30,31 (2006).
ADA activity holds significant diagnostic value for pericardial tuberculosis.32 Despite being the most commonly used biochemical test, ADA has limitations due to its variability, and the yields from fluid microscopy and culture remain both low and time-consuming.5,33 Consequently, in many regions with high TB burden, empirical treatment is often practiced, even though evidence suggests that such empiric therapy may lead to increased morbidity and mortality.5 ADA levels ≥35 U/L in pericardial fluid are considered diagnostic for TP, with a sensitivity of 90% and specificity of 74%, respectively.2,34 However, in the present study, ADA was not significantly associated with TP patients (p value- 0.1576). A similar finding has been reported by Deepinder Chhinna et al.30 (2013).
TB is typically linked with very high ESR values (≥100 mm/h),35 but in this study, ESR did not show a significant association with TB (p-value = 0.7176).
TP is associated with a high mortality rate, ranging from 17% to 40%.2 In the present study also the mortality rate is 33%.
CONCLUSION
Use of molecular diagnosis may offer early, specific diagnosis. Despite poor sensitivity GeneXpert MTB/RIF can aid in ruling out tubercular etiology. Lymphocyte count, ADA level, and ESR were not found to be significantly associated with TP. Hence, we advocate the use of molecular methods recommended by the WHO for early diagnosis of TP, which will also reduce sinister outcomes like mortality.
Authors’ contributions
PR, KB, JB, JA, RB, VSY, RN, US: Each author contributed in designing the work, revising it and final approval of the work.
Ethical approval
The research/study approved by the Institutional Ethics Committee at All India Institute of Medical Sciences, reference No.- 743/22, dated, 02nd February 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Extrapulmonary tuberculosis. Expert Rev Respir Med. 2021;15:931-48.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculous pericarditis-a silent and challenging disease: A case report. World J Clin Cases. 2022;10:1869-75.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Lessons on the quality of tuberculosis diagnosis from standardized patients in China, India, Kenya, and South Africa. J Clin Tuberc Other Mycobact Dis. 2019;16:100109.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Informe eventotuberculosis a periodo XI-2020. Bogotá: Instituto Nacional de Salud; 2020. Available from: https://www.ins.gov.co/buscador-eventos/Lineamientos/PRO_Tuberculosis.pdf [Last accessed 2021 May 10]
- Diagnosis and management of tuberculous pericarditis: What is new? Curr Cardiol Rep. 2020;22:2.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology of pericardial diseases in africa: A systematic scoping review. Heart. 2019;105:180-8.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculous pericarditis—own experiences and recent recommendations. Diagnostics. 2022;12:619.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tuberculous pericardial disease: A focused update on diagnosis, therapy and prevention of complications. Cardiovasc Diagn Ther. 2020;10:289-95.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Kent PT, Kubica GP, eds. Public health mycobacteriology: A guide for the Level III laboratory. Atlanta, GA: Centers for Disease Control; 1985. Available online at: https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB86216546.xhtml [Last accessed 2024 Aug 02]
- TB India 2020. RNTCP. Central TB Division, DGHS. Ministry of Health & Family Welfare; Available online at: https://tbcindia.gov. in/ [Lat accessed 2020 Nov 14]
- Bain BJ, Bates I, Laffan MA, eds. Dacie and lewis practical haematology (12th edition). London, UK: Elsevier; 2017.
- Treatment of tuberculosis in complex emergencies in developing countries: A scoping review. Health Policy Plan. 2018;33:247-57.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation and treatment of pericarditis: A systematic review. JAMA. 2015;314:1498-506.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences in tuberculosis. Semin Immunopathol. 2019;41:225-37.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601-6.
- [CrossRef] [PubMed] [Google Scholar]
- Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr Probl Cardiol. 2013;38:7-46.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Gonadectomy of male BALB/c mice increases tim-3(+) alternatively activated M2 macrophages, tim-3(+) T cells, Th2 cells and treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav Immun. 2009;23:649-57.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68:5126-32.
- [CrossRef] [PubMed] [Google Scholar]
- Demographic, clinical and etiological profile of pericardial effusion in india: A single centre experience. Indian J Tuberc. 2022;69:220-26.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic massive pericardial effusion: A case report and literature review. J Int Med Res. 2020;48:300060520973091.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An approach to the patient with suspected pericardial disease. S Afr Med J. 2016;106:151-5.
- [CrossRef] [PubMed] [Google Scholar]
- Global, regional, and national sex differences in the global burden of tuberculosis by HIV status, 1990-2019: Results from the global burden of disease study 2019. Lancet Infect Dis. 2022;22:222-41.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tuberculous pericardial disease: A focused update on diagnosis, therapy and prevention of complications. Cardiovasc Diagn Ther. 2020;10:289-95.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diagnostic test accuracy of xpert MTB/RIF for tuberculous pericarditis: A systematic review and meta-analysis. F1000Res. 2020;9:761.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Inhibition mechanisms of hemoglobin, immunoglobulin g, and whole blood in digital and real-time PCR. Anal Bioanal Chem. 2018;410:2569-83.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Circumventing qPCR inhibition to amplify miRNAs in plasma. Biomark Res. 2014;2:13.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Determinants of PCR performance (Xpert MTB/RIF), including bacterial load and inhibition, for TB diagnosis using specimens from different body compartments. Sci Rep. 2014;4:5658.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Lymphocyte subpopulations in pulmonary tuberculosis patients. Mediators Inflamm. 2006;2006:89070.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Early diagnosis in an unusual presentation of tubercular pericarditis-A case report. Asian Pac J Trop Dis. 2013;3:161-3.
- [CrossRef] [Google Scholar]
- tuberculous pericarditis and myocarditis in adults and tuberculosis E-Book: A Comprehensive clinical reference. Amsterdam, The Netherlands: Elsevier; 2009. p. :351.
- Diagnostic accuracy of adenosine deaminase for tuberculous pericarditis: A meta-analysis. Eur Rev Med Pharmacol Sci. 2015;19:4411-8.
- [PubMed] [Google Scholar]
- Diagnostic values of xpert MTB/RIF, t-SPOT.TB and adenosine deaminase for HIV-negative tuberculous pericarditis in a high burden setting: A prospective observational study. Sci Rep. 2020;10:16325.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Erythrocyte sedimentation rate values in cases of active tuberculosis without HIV co-infection. J Med Sci Clin Res. 2016;4:13156-9.
- [CrossRef] [Google Scholar]






