Translate this page into:
A rare incidence of cerebral venous thrombosis in a case of immune thrombocytopenia on eltrombopag
*Corresponding author: Kundan Mishra, Department of Hematology, Army Hospital (Research and Referral), New Delhi, India. mishrak20@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mishra K, Barki S, Sreen A, Saravagi G, Kumar S. A rare incidence of cerebral venous thrombosis in a case of immune thrombocytopenia on eltrombopag. Ann Natl Acad Med Sci (India). 2024;60:225-7. doi: 10.25259/ANAMS-2023-9-14-(1042)
Abstract
Immune Thrombocytopenia (ITP) typically presents with mucocutaneous bleeding. Splenectomy, rituximab, and thrombopoietin receptor agonists (TPO-RAs) are the recommended second-line therapy. Eltrombopag is one of the TPO-RAs used for the treatment of steroid-refractory ITP, with a response rate of 70–80%. Though rare, yet various cases of venous thromboembolism have been reported from clinical trials as well as real-world studies. We present a case of ITP that was refractory to corticosteroid and second-line drugs; however, it responded to eltrombopag. While having a complete response on eltrombopag, the patient developed Cerebral Venous Thrombosis.
Keywords
Cerebral venous thrombosis
CVT
Eltrombopag
Immune thrombocytosis
ITP
INTRODUCTION
Immune Thrombocytopenia (ITP) is a diagnosis of exclusion. Clinically, it is characterized by low peripheral blood platelet (<100,000/cmm) and typically presents with mucocutaneous bleeding.1,2 The first-line therapy consists of corticosteroids with or without immunoglobulins with a response rate of 70–90%.3 However, the majority of patients have a loss of response and require second-line therapy. Splenectomy, rituximab, and thrombopoietin receptor agonists (TPO-RAs) are the recommended second-line therapy.4–6 Eltrombopag is one of the TPO-RAs used for the treatment of steroid-refractory ITP, with a response rate of 70–80%.7 Though rare, yet various cases of venous thromboembolism (VTE) have been reported from clinical trials as well as real-world studies. Cerebral venous sinuses are an uncommon and rare site of thrombosis. There are limited numbers of case reports of cerebral venous thrombosis (CVT) in the literature and all are from the Western world.8 We present a case of refractory ITP that developed CVT while on eltrombopag therapy.
CASE REPORT
A 27-year-old lady was diagnosed with acute ITP in September 2021. Her Antiphospholipid Antibody (APLA) antibodies were negative. She received dexamethasone 40 mg once daily for four days without any response. Due to wet purpura, she was given immunoglobulin with transient improvement in platelet. In view of no response with a corticosteroid, she was started on romiplostim 3 mcg/kg. The dose was increased to 10 mcg/kg (1 mc/kg increase every week) with no response. Due to persistent disease and poor response, she underwent an extractable nuclear antigen test (ENA), which was negative. She was given rituximab 375 mg/m2 weekly × four doses without any response. She was started on eltrombopag 50 mg once daily (empty stomach) and increased to 75 mg OD (in view of no response after four weeks). Eight weeks on eltrombopag 75 mg OD, she had shown response with platelet counts increasing up to 36,000/cmm. She was continued on eltrombopag 75 mg OD.
Two months later, she reported to emergency with acute onset severe headache, which gradually progressed over the last three days. She took some over-the-counter painkiller pills without any relief. She also had multiple episodes of projectile vomiting. On ophthalmoscopy, she had grade two papilloedema, and an urgent Non-contrast Computed Tomography (NCCT) head was suggestive of left parieto temporal SAH with an extension into the ventricle. She was admitted to the intensive care unit and treated with mannitol, levetiracetam, and supportive care. Her blood reports were hemoglobin 9.3 g/dL, MCV 67.5fL WBC 19500/cmm, and platelet 2.91 L/cmm. In view of a rapid increase in platelets since the last follow-up and no risk factors for subarachnoid hemorrhage, a clinical diagnosis of CVT was contemplated. She was taken up for cerebral angiography. The CT angiography images of the brain showed extensive venous thrombosis [Figure 1]. The findings were confirmed on MR images of the brain [Figure 2]. There was no family history of VTE. She was started on enoxaparin 1 mg/kg SC twice daily, and the supportive care continued. However, she had a rapidly worsening course in the hospital and succumbed to her illness.
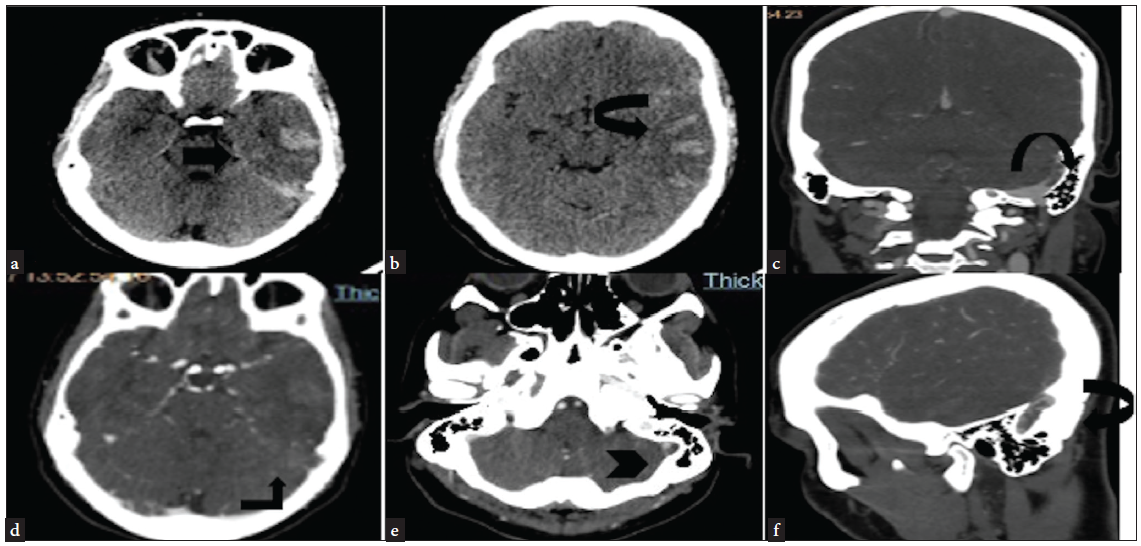
- NCCT head (a and b) axial section—hyperdense contents (black arrow) (suggestive of SAH) involving the sylvian fissure and sulcal spaces and parenchyma of the left temporoparietal lobe. (c, d, e, and f)—CT angiography second phase—hypodense partially occlusive patchy and linear areas of filling defects suggestive of thrombus seen in the left sigmoid sinus (black arrowhead in e). NCCT: Non-contrast computed tomography, SAH: Sub-arachnoid hemorrhage.
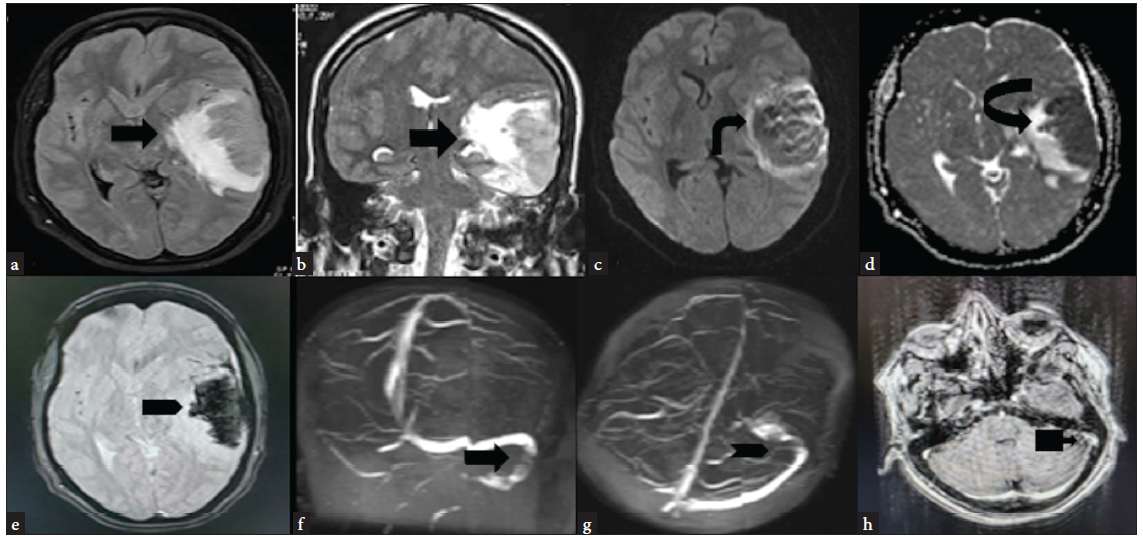
- MRI (a and b) FLAIR axial and T2 coronal—hyperintense area of altered signal intensity involving the cortex and underlying white matter of the left temporoparietal lobe (black arrow). (c and d) DWI and ADC maps—lesion is showing a patchy area of restriction of diffusion with corresponding reduced ADC values (black arrow). (e) GRE axial—diffuse are of signal loss/blooming as seen suggestive of intraparenchymal hemorrhage (black arrow). (f and g) 2D TOF MRV coronal and axial—hypointense filling defects with peripheral hyperintensity within the left sigmoid sinus (black arrow) (black arrowhead). (h) Gadolinium-enhanced T1 Axial—central filling defect in left sigmoid sinus with peripheral post-contrast enhancement (black arrow). FLAIR: Fluid-attenuated inversion recovery, DWI: Diffusion-weighted imaging, ADC: Apparent diffusion coefficient, GRE: Gradient recalled echo, TOF: Time-of-flight, MRV: Magnetic resonance venography.
DISCUSSION
CVT in steroid-refractory ITP on eltrombopag is rare, and so far, only eight cases have been reported in the literature.8–13 Our case is only the second from India and overall ninth in the world. Eltrombopag increases the platelet by direct action on megakaryocytes as well as immunomodulation by acting on T-regulatory cells. Though it has a very good safety profile, VTE has been reported from the clinical trials initially and real-world case reports subsequently. The sudden rise in young platelets, which are bigger in size and have more granular cytoplasms, predisposes to thrombus formation at various venous beds. This may pertain to the better platelet function reported in cases of ITP preventing bleeding despite having critically low platelet14,15 Though CVT is rare, it results in severe morbidity and rarely results in death. To prevent this situation, we, in our clinical practice, start patients on ecosprin once the patient achieves complete response (platelet >100,000/cmm) on eltrombopag. However, a multicenter trial is required to evaluate the benefit and safety of such preventive intervention. The index case emphasizes that for any patient of ITP on eltrombopag presenting with unexplained headache, focal neurological deficit, or neuroimaging suggestive of ischemic changes with or without hemorrhage, CVT must be considered as one of the possibilities, and prompt anticoagulation can be lifesaving.
CONCLUSION
CVT is a rare but life-threatening complication in ITP patients on eltrombopag. High degree of suspicion is required for early diagnosis and therapy.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Update on diagnosis and treatment of immune thrombocytopenia. Expert Rev Clin Pharmacol. 2021;14:553-68.
- [CrossRef] [PubMed] [Google Scholar]
- Wet purpura: A sinister sign in thrombocytopenia. BMJ Case Rep. 2017;2017:bcr2017222008.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Real-world experience of anti-D immunoglobulin in immune thrombocytopenia. Ann Hematol. 2022;101:1173-9.
- [CrossRef] [PubMed] [Google Scholar]
- American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3:3829-66.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Safety and efficacy of splenectomy in immune thrombocytopenia. Am J Blood Res. 2021;11:361-72.
- [PubMed] [PubMed Central] [Google Scholar]
- Real-world experience of rituximab in immune thrombocytopenia. Indian J Hematol Blood Transfus. 2021;37:404-13.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Real-world experience of eltrombopag in immune thrombocytopenia. Am J Blood Res. 2020;10:240-51.
- [PubMed] [PubMed Central] [Google Scholar]
- Cerebral venous sinus thrombosis in immune thrombocytopenia patients treated with thrombopoietin receptor agonist: Case reports and literature review. Ann Med Surg (Lond). 2022;79:104116.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Eltrombopag-associated cerebral venous thrombosis. Am J Ther. 2021;28:e167-e9.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent cerebral venous sinus thrombosis secondary to eltrombopag for ITP. J Neurol Neurosurg Psychiatry. 2016;87:e1.
- [Google Scholar]
- Extensive cerebral venous sinus thrombosis following a dose increase in eltrombopag in a patient with idiopathic thrombocytopenic purpura. Platelets. 2014;25:144-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral venous thrombosis in a patient with immune thrombocytopenia, an apparent paradox. Case Reports in Oncology. 2020;13:588-94.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cerebral venous thrombosis in refractory idiopathic thrombocytopenia treated with eltrombopag. Ann Indian Acad Neurol. 2016;19:532-3.
- [CrossRef] [PubMed] [Google Scholar]
- Poor platelet function on sonoclot signature is associated with high incidence of bleeding in severe immune thrombocytopenia. Blood. 2018;132:4991.
- [Google Scholar]
- Re: Risk factors and predictors of treatment responses and complications in immune thrombocytopenia. Ann Hematol. 2021;101:447-8.
- [CrossRef] [PubMed] [Google Scholar]





