Translate this page into:
NAMS task force report on Venous thromboembolism
*Corresponding author: Lt Gen (Dr.) Velu Nair, Chairperson, VTE Task Force, National Academy of Medical Sciences (India), India. Email: nairvelu2000@yahoo.com; nams_aca@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: National Academy of Medical Sciences (India). NAMS task force report on Venous thromboembolism. Ann Natl Acad Med Sci (India). 2024;60:34–70. doi: 10.25259/ANAMS_TFR_01_2024
INTRODUCTION
Venous thromboembolism (VTE) comprises deep vein thrombosis (DVT) and pulmonary embolism (PE). It can result in significant mortality, morbidity, and healthcare costs. Approximately 30% of patients with symptomatic VTE manifest with PE, and others with DVT. The annual incidence of PE ranges from 39 to 115 per 100,000 population, and for DVT, the incidence ranges from 53 to 162 per 100,000 population. VTE not only disables patients but also prolongs hospital stay, leading to an increase in the cost of treatment.
PE is one of the most common causes of sudden unexplained deaths in hospitalized patients. An early diagnosis and prompt, effective treatment is crucial, as the mortality rate of untreated PE is about 30%, and nearly 30% of untreated DVTs suffer severe swelling or ulceration of the lower limbs. With timely diagnosis and treatment, PE-related deaths are less than 1%. Venous thrombosis (VT) generally lacks specific signs and symptoms, which can lead to a delayed or inaccurate diagnosis and inferior patient outcomes. Hence, awareness creation amongst treating physicians is a key approach to combating VTE.
The most common presentations of VT are DVT of the lower extremity and PE. These can result in significant mortality, morbidity, and healthcare costs. The causes of VT can be divided into two groups: hereditary and acquired, and are often multiple in a given patient. A major theory delineating the pathogenesis of VTE, often called Virchow Triad, proposes that VTE occurs as a result of:
Alterations in blood flow (i.e., stasis)
Vascular endothelial injury
Alterations in the constituents of the blood (i.e., inherited or acquired hypercoagulable state)
A risk factor for thrombosis can be identified in over 80% of patients with VT. Furthermore, there is often more than one factor at play in a given patient. Accordingly, many patients with VTE fulfill most or all of Virchow’s triad of stasis, endothelial injury, and hypercoagulability. As examples:
Fifty percent of thrombotic events in patients with inherited thrombophilia are associated with the additional presence of an acquired risk factor (e.g., surgery, prolonged bed rest, pregnancy, oral contraceptives). Some patients have more than one form of inherited thrombophilia or more than one form of acquired thrombophilia and appear to be at even greater risk for thrombosis.
In a population-based study of the prevalence of VTE, 56% of the patients had three or more of the following six risk factors present at the time of VTE: > 48 hours of immobility in the preceding month; hospital admission, surgery, malignancy, or infection in the past three months; or current hospitalization.
BACKGROUND
The incidence of DVT in India in the general population is about 1.79 per thousand. Approximately 30% of patients with symptomatic VTE have PE, and others have DVT. More than 50% of post-surgical procedure patients are at risk of developing VTE. The risk of VTE following total knee arthroplasty (TKA) is 72.2%, following abdominal or thoracic surgeries is 40%, and following total hip arthroplasty (THA) is 42.9%. Idiopathic DVT is noted in 43% of patients, with 52% of patients developing DVT after a precipitating event and 6% of patients showing recurrence of DVT. The incidence of DVT after major lower limb surgery in Indian patients is comparable to Western data.
Increasing age, male gender, trauma, surgery, prolonged hospitalization, malignancy, neurologic disease, central venous catheter, prior superficial vein thrombosis, and varicose veins have been identified as some of the major risk factors for developing VTE. In women, oral contraceptive pill use, pregnancy, and hormone replacement therapy have been established as independent risk factors. Some of the important risk factors for surgical patients developing VTE are age, type of surgery, length of procedure, and duration of immobilization.
VTE is a major cause of cardiovascular morbidity and mortality and has a known genetic contribution. Genetic risk factors predispose to thrombophilia and play the most important etio-pathogenic role in VTE in people younger than 50 years. At least one inherited risk factor could be found in about half of the cases with a first episode of idiopathic VTE. A revolutionary contribution to the genetic background of VTE was brought by the achievements of the genome-wide association studies which analyze the association of a huge number of polymorphisms in a large sample.
The detection of hereditary thrombophilia has an impact on the management of the anticoagulation in children with purpura fulminans, and pregnant women at risk of VTE and may be useful in the assessment of the risk for recurrent thrombosis in patients presenting an episode of VTE at a young age (<40 years) and in cases with positive family history regarding thrombosis. Data showing the clinical usefulness and benefits of testing are limited or nonexistent, as are data supporting the benefit of primary or secondary VTE prophylaxis based on thrombophilia status alone. Patients with inherited thrombophilia can often be identified by coagulation experts on the basis of the patient’s personal and family history of VTE, even without knowledge of test results. Factors associated with the presence of an inherited thrombophilia include VTE at a young age (often considered to be less than 40–50 years of age); a strong family history of VTE, VTE in conjunction with weak provoking factors at a young age; recurrent VTE events; and VTE in an unusual site such as the central nervous system or splanchnic veins.
While acute precipitants and clinical risk factors are often the focus of determining the cause of VTE, a small minority of patients have a mutation in a limited number of genes leading to an inherited thrombophilia. To that end, hypercoagulability and/or genetic testing can identify some uncommon genetic mutations such as factor V Leiden, antithrombin deficiency, protein C or S deficiency, or a prothrombin gene mutation. However, standard testing is usually unrevealing, with mutations present in only about 5% of the general population. Thus, for many patients with VTE, no clear precipitant or risk factor is ever identified.
When a patient experiences a VTE event without an acute precipitant such as recent surgery, immobilization, or trauma, one often considers clinical risk factors and contemplates testing for a handful of known monogenic thrombophilia disorders. However, the use of thrombophilia testing has fallen out of favor in part due to the low yield in terms of the number of patients identified. Given the genetic backdrop, the studies done in India have certain limitations, which include but are not limited to sample size being quite limited and the studies having been done in the context of VTE with some other co-morbidity. Further, mostly targeted polymorphisms have been analyzed, with no study involving a global approach at the genome-wide level. Indian data depict that the established thrombophilia genetic markers Factor V Leiden and Prothrombin G20210A have a limited role from the Indian perspective. Several studies have shown the role of Factor V Leiden in VTE risk but only with certain comorbidity in the Indian population. In recently published landmark studies regarding the genetics of VTE in the journals Blood and Nature Genetics, in contrast to uncommon thrombophilias, genome-wide association studies were used to identify 297 independent single nucleotide polymorphisms associated with VTE, from which a polygenic risk score was developed. These data demonstrate that consideration of broader polygenic risk can identify a much larger proportion of patients at risk for VTE and is a stronger predictor than many chronic clinical risk factors. We need to extend these kinds of studies in the Indian population to get a comprehensive genetic view on VTE.
Preventing fatal PE is the primary goal of anticoagulant prophylaxis for VTE. Prevention of VTE also avoids significant post-VTE morbidity. Conditions that can develop despite appropriate treatment of VTE are post-thrombotic syndrome (PTS), chronic thromboembolic pulmonary hypertension (CTEPH), and post-PE syndrome. The prevalence and potential severity of these conditions must be considered when determining the potential benefits of preventing VTE. Averting sudden death and reducing post-PE morbidity are not the only benefits of anticoagulant prophylaxis, and prevention of VTE is important to avoid patient discomfort, anticoagulant treatments and their associated risks, specialist visits, delays in procedures, and the potential for additional testing.
Terms of Reference for the Task Force
The Executive Council of the National Academy of Medical Sciences (India) had assigned the following terms of reference for the Task Force (TF) on VTE in April 2022. All recommendations of the TF were to be placed before the Executive Council of the NAMS by the end of July 2022, for approval and onward submission to the Government of India.
The TF was required to make recommendations to the Government of India for the prevention and control of VT and embolism in India at the health policy and implementation levels.
The TF would prepare a “White Paper” which may include the existing morbidity and mortality status, if available due to VT and thromboembolism.
The TF would identify existing lacunae and deficiencies in the thematic area and make recommendations to address these.
METHODOLOGY
On receipt of the terms of reference from the NAMS Executive Council, the TF was convened under the Chairmanship of Lt Gen Velu Nair with membership from a cross-section of domain experts enlisted in the task force.
Through a process of discussions in the virtual mode, a consensus was reached among the members of the TF, on the methodology to be adopted for developing ibid guidelines. The task at hand was divided into sections, and members allocated the sections based on their specific domain expertise.
An extensive literature review was undertaken using the websites PubMed and Google Scholar using the search terms “Venous Thromboembolism” AND “Management” AND “Prophylaxis” AND “Prevention” for English language documents, with a preference for review articles, clinical trials, consensus statements and guidelines. Professional society websites were browsed for the latest guidelines and consensus statements. Contribution from the scientific committee was requested through personal communication from the Chairperson to all members of the Indian Society for Hematology. Thus, almost all published work from India was reviewed along with all similar international work on VTE. A synthesis of the obtained literature was prepared and deliberated upon by the TF.
A series of weekly meetings were conducted in virtual mode for reviewing the progress being made and to discuss the allocated sections of the White Paper. Minutes of the meetings were prepared and circulated within the TF for information and guidance.
While developing the document, the PICO framework was relied upon to define the various at-risk patient groups and recommend the interventions required. Iterations of the document developed with the contributions of the members were circulated and discussed sequentially over the term of the TF. This modification of the Delphi technique was essential for the process of eventual consensus, as the guidelines required reference to the latest evidence and conformity with professional society guidelines, keeping in view the requirements of the country and the best interests of the patient population.
SITUATIONAL ANALYSIS
Current situation in India
The prevailing notion that the incidence of VTE in Asians is less than that in the Western population has been disproved by recent reports. The incidence of postoperative DVT in Indian patients undergoing major lower limb surgery is as high as seen in the Western world (43.2% and 60% of patients in the groups with and without prophylaxis, respectively). In a study covering 549 patients, acute DVT without PE, acute DVT with PE, and PE alone were reported in 64%, 23%, and 13% of patients, respectively. The mean age was 47 (±16) years, and 70% of the patients were males. A history of DVT (34%), surgery including orthopedic surgery (28%), trauma (16%), and immobilization >3 days (14%) were the most common risk factors for VTE. Hypertension (25%), diabetes (19%), and neurological disease (other than stroke) (8%) were the most common comorbidities. Most (94%) were treated with heparin alone (82%) or fondaparinux (2%) for initial anticoagulation; low molecular weight heparin alone (5%) or warfarin/acenocoumarol (76%) for long-term anticoagulation.
In the MEGA study, patients who were tested for thrombophilia after a first episode of VTE were analyzed for the outcomes of testing and for reduction in the risk of recurrence. It was observed that despite thrombophilia testing at the time of first VTE, 35% of patients had recurrent VTE during follow-up compared with 30% of patients who did not have recurrent VTE. This indicated that testing at the time of the first VTE did not reduce the risk of recurrence of VTE. Testing for inherited thrombophilia does not reduce the recurrence of VT. The recurrence risk for VTE is determined by the clinical situation (e.g., provoked vs. unprovoked) along with non-Mendelian risk factors (e.g., body mass index and age) rather than the inherited thrombophilia panel.
The proportion of Indian patients at risk for VTE (53.6%) was similar to that of the global patients at risk for VTE (51.8%). However, ENDORSE data showed that globally, 50.2% of at-risk patients received ACCP-recommended prophylaxis, whereas in India, only 17.4% of at-risk patients received such prophylaxis. Among at-risk patients, 18.5% of surgical and 22.4% of medical patients received any VTE prophylaxis. Similarly, 16.3% of surgical and 19.1% of medical patients received ACCP-recommended thromboprophylaxis. In a prospective registry on venous thromboembolic events (PROVE) conducted in 19 countries, 3,526 patients with symptomatic DVT were enrolled, out of which 667 were from India. Prior VTE prophylaxis had been given to only 5% of enrolled Indian patients compared to 12% in the overall PROVE population.
Thus, thromboprophylaxis in India may not be routine practice in most institutions other than tertiary care hospitals. This large population of patients at-risk for VTE identifies an unmet need. There is thus a need to understand the incidence and prevalence of VTE in various medical and surgical settings better.
The most common reasons for the underutilization of pharmacological thromboprophylaxis are varied and often include (but are not limited to) lack of knowledge, ignorance, fear of bleeding, and any contraindication to anticoagulants. This can be interpreted to imply poor awareness of the risks of VTE in patients. The current paradigm for diagnosis and management of thrombosis has provided a variety of tools. However, it has also left some unanswered questions, such as methods for risk stratification to predict the risk for recurrent VTE requires aggressive anticoagulation.
Current infrastructure, facilities, technologies, policies, programs, etc., in India in the context of the problem of VTE
The ICMR has recently launched a National Hospital-based Registry on Venous Thromboembolic Disorders (i-RegVeD) with the aim of establishing a nationwide surveillance network through selected hospitals for the collection of data for generating evidence on VTE prevalence. This will be of relevance for planning suitable, calibrated responses and strengthening healthcare facilities across different treatment settings. This registry is based on a standard reporting framework with data capture using electronic information technology for timely analytics of patterns of disease distribution, treatment, and outcomes of VTE patients. The data are intended to be used for relevant and appropriate research and innovation, including identifying risk factors for VTE disease. It is anticipated that the registry shall contribute to improving patient management for VTE and related manifestations, and also guide policy and health planning in the future.
Presently, there is no formal campaign or system for the upgradation and verification of skill sets of healthcare professionals in managing and prescribing prophylaxis for VTE. Online consensus statements and guidelines are available from international professional societies which can be accessed by any interested healthcare professional; however, there is no focused initiative to regulate or standardize the approach across the Indian healthcare system.
Across the healthcare hierarchy in India, in the governmental public healthcare system, the availability of laboratory diagnostic capabilities and pharmaceutical supplies for managing, monitoring, and providing prophylaxis for VTE is not uniform. This problem is especially acute at the peripheral levels of the healthcare system, from the district hospital downward, compounded by the problem of a relative lack of trained and specialist human resources. In the private healthcare system, which accounts for the majority of tertiary healthcare sought by the Indian population with considerable out-of-pocket expense being incurred, the management of VTE among other conditions is dependent mostly on perceived commercial considerations.
With the Indian Public Health Standards being formulated to address the human resource and equipment needs across a standard template and scale, in an ideal scenario, the requirements of trained human resources have been addressed. However, ground reality indicates otherwise, with scorecards from the Niti Aayog revealing the realities. So far, there has been no comprehensive assessment of the competence per se of such trained personnel across a spectrum of clinical domains, leave alone VTE as a focus area.
The National Essential Diagnostics List was promulgated in 2019, with states being empowered to augment the list and provide equipment to suit their specific inclinations or areas of focus in disease and health management. This guideline serves to provide a generic template for states to plan service delivery. The framework for VTE management thus can capture the parameters outlined in this document to provide support to states in planning their response.
Key issues/gaps identified in the context of VTE
These include but are not limited to the following:
Trained human resources (healthcare professionals): Availability of an adequate number of personnel adequately oriented toward VTE and skilled suitably to manage VTE in different clinical settings and provide thromboprophylaxis as required.
Laboratory diagnostic support: Facilities that are commensurate with the basic minimum requirements to screen for, diagnose, and manage VTE at different levels of the healthcare system.
Availability of pharmaceutical supplies: Appropriate drug stocking and replenishment systems, including required logistics, for the healthcare facilities to provide appropriate care and manage VTE at different levels of the healthcare system.
Awareness in the community: Awareness about VTE and the risks posed to health. To inculcate required healthcare-seeking behavior with specific reference to prevention and early recognition.
Research and future direction: To study the incidence and prevalence in the Indian population and undertake research to identify suitable protocols for screening, diagnosis, and management appropriate for the country.
Equitable distribution of facilities for management of VTE: Availability of healthcare facilities that are adequate and accessible, affordable and sustainable, appropriate and acceptable in the geographical vicinity of at-risk population clusters.
RECOMMENDATIONS
Vision
It is recommended that consensus be achieved amongst the various stakeholders in the health of the people of India toward a national vision, that is, a venous thromboembolism-free healthcare system and eventually a VTE-free population.
The National Academy of Medical Sciences (India) envisions a healthcare system in India, both in the public sector and the private healthcare system, in which a collaborative, multidisciplinary approach will ensure a VTE-free population and a VTE-free healthcare system through standardized, evolving, evidence-based guidelines, to deliver sustainable, high-quality, affordable, and patient-focused care.
Further to National Health Policy 2017, the goal of a VTE-free healthcare system and VTE-free population, being proposed by the National Academy of Medical Sciences (India), is for the attainment of the highest possible level of health and well-being for all, at all ages, through a preventive and promotive healthcare orientation, and universal access to good-quality healthcare services without anyone having to face financial hardship as a consequence, by eliminating incidence of VTE amongst other initiatives of the Government of India.
Recommendations made to bridge critical gaps/deficiencies
Presently, thromboprophylaxis as an approach in the management of patient populations at risk is underutilized in India; hence, measures to overcome this unmet need are warranted [Annexure 1]. There is also a need to have focused, simple guidelines with algorithms and charts for care providers for early diagnosis of VTE and prompt management in different VTE settings (including at the community level). In addition [Annexures 2–5], it is important to increase awareness among treating physicians regarding guidelines on testing for VTE, while avoiding unnecessary testing. These need to be undertaken in the backdrop of promoting health literacy on a broader palette for the larger population [Annexure 6].
The various recommendations of the NAMS TF include but are not limited to the following Table 1
| Key Focus areas/Gaps | Action Recommended |
|---|---|
| Trained human resources | Periodically updated online training modules be made available for healthcare personnel nationally, with regional mentoring by medical institutions of eminence. |
| Laboratory diagnostic support | The National Essential Diagnostics List 2019 and the relevant facility-wise Indian Public Health Standards be utilized to standardize the laboratory requirements for basic minimum requirements to screen for, diagnose, and manage VTE at different levels of the healthcare system. |
| Availability of pharmaceutical supplies | States be advised to refine their Essential Drugs List to include/retain appropriate drugs and ensure stocking and replenishment systems, including required logistics, for their healthcare facilities to provide appropriate care and manage VTE at different levels of the healthcare system. |
| Awareness in the community | Periodic campaigns be launched regionally with standard content about VTE and the risks posed to individual health. Behavior change campaigns may also be promoted to inculcate required healthcare-seeking behavior with specific reference to prevention and early recognition of VTE. |
| Research and future direction | Funding be made available to study the incidence and prevalence in the Indian population and undertake research to identify suitable protocols for screening, diagnosis, and management appropriate for the country. |
| Equitable distribution of facilities for the management of VTE | State governments may be advised to plan for the availability of healthcare facilities that are adequate and accessible, affordable and sustainable, appropriate and acceptable in the geographical vicinity of at-risk population clusters. |
NAMS: National Academy of Medical Sciences (India); TF: Task Force; VTE: venous thromboembolism.
WAY FORWARD
The NAMS TF, after due deliberations on the need for current evidence about thromboprophylaxis practices has proposed the conduct of a rapid multicentric cross-sectional study on a pan India basis to ascertain a representative view of the VTE burden and real-world prophylaxis practices. This is intended to be undertaken to develop indigenous VTE risk assessment tools and prophylaxis strategies.
Efficient use of healthcare resources is extremely crucial, and diagnostic testing without significant clinical utility is not recommended as per multiple specialty societies, including the current ACCP and ASH guidelines. There are two major ways to reduce the risk of VTE. The first is to screen patients pre- and postoperatively with accurate diagnostic testing. By diagnosing VTE early, treatment could be provided to halt progression and avoid morbidity and mortality associated with acute VTE. Unfortunately, contrast venography is expensive, painful, and impractical to perform outside of clinical studies. Less invasive studies, such as venous ultrasonography (US), are less sensitive in asymptomatic patients than in symptomatic patients. Screening “at-risk” patients is impractical and too expensive to be undertaken outside of clinical trials.
The second approach is to undertake measures to prevent VTE. General measures, such as encouraging early ambulation after surgery, can be adopted universally without harm. In addition, active prophylaxis with either mechanical or pharmacologic means has been proven to lower the risk of VTE. Mechanical prophylaxis refers to devices, such as graduated compression stockings and intermittent pneumatic compression devices, which decrease venous stasis in the lower extremities. Mechanical prophylaxis does not carry a risk of bleeding but can be uncomfortable, and prolonged use can lead to skin breakdown and other cutaneous complications.
Suggested policy activities and advocacy for policy makers
DVT and PE have been recognized to be major public health problems across the world today. Clinicians and hospitalists are assumed to know how to reduce the morbidity and mortality resulting from DVT/PE, yet it is perceived that this knowledge is mostly not being applied systematically at the population level or even uniformly across healthcare facilities [Annexure 7]. Without a concerted effort to stem the public health crisis that unrecognized VTE poses, the incidence and burden of these diseases will only grow larger as the population in India ages.
The key actions required to be taken by policymakers in various settings have been given in Annexures 8, 9.
The recently launched ICMR registry for VTE is recommended to be actively promoted for voluntary participation by healthcare facilities across the country.
Recommendations for healthcare professionals
It is proposed that a focused, continuing professional education campaign be conceptualized and launched, targeting healthcare professionals. An outline of such an educational module is given in Annexure 8. This is proposed to be done in tandem with a suitably structured patient and community-focused campaign.
Suggestions to create awareness among general public, NGOs, and community stakeholders
A community-based strategy is recommended to create awareness periodically [Annexure 6]. This is recommended to be steered by a special committee or cell that may be established by the Ministry of Health and Family Welfare, with the cooperation of the Indian Society of Hematology in coordination with the Indian Public Health Association.
The specific areas of focus of community-focused campaigns may include but not be limited to the following:
All about healthcare-associated VTE, including risk factors
All about blood clots and travel
All about blood clots and pregnancy
All about blood clots and cancers.
The intent would be to provide information as appropriate, with a regional flavor, and to promote healthcare-seeking behavior on a broader palette of behavior change communication aimed at health literacy.
Areas of future research
In the current scenario, establishing a thrombophilia testing setup in the Indian population is difficult as there is a relative lack of well-designed, population-based studies that could associate genetic risk factors with disease prevalence. A large-scale population-based genome-wide association study is thus essential to identify the genetic associations with VTE. Details are further outlined in Annexure 10.
DOCUMENTS REFERRED BY THE TF
Technical documents from various professional societies, such as the ASH, ACCP, etc., were perused. Apart from these, landmark peer-reviewed articles over the past two decades were also reviewed.
“Suggested further reading,” includes some of the relevant articles/documents referred to by the TF.
ANNEXURE 1
Annexure 1 briefly outlines the task force VTE prophylaxis recommendations through Tables I– XV appended below
VTE PROPHYLAXIS: RECOMMENDATIONS
| Medical patients | |||
|---|---|---|---|
| Risk stratification | Score | Choice of VTE prophylaxis | Remarks |
|
IMPROVE VTE1 IMPROVE Bleeding Risk Score2 |
IMPROVE VTE score < 3 |
Nil | |
|
IMPROVE VTE score ≥ 3 & IMPROVE Bleeding risk score < 7 |
LMWH UFH or Fondaparinaux |
If
UFH can be used as an alternative
|
|
|
IMPROVE VTE score ≥ 3 & IMPROVE Bleeding risk score ≥ 7 |
Mechanical prophylaxis with graduated compression stockings or intermittent pneumatic compression | Switch to pharmacologic prophylaxis once the bleeding risks return to normal. | |
IMPROVE: International Medical Prevention Registry on Venous Thromboembolism; VTE: Venous thromboembolism; LMWH: Low molecular weight heparin; UFH: Unfractionated heparin; CrCl: Creatinine clearance; DOAC: Direct oral anticoagulant.
Source: 1Spyropoulos AC, et al. Chest 2011 Sep;140(3):706-714; 2Decousus H, et al. Chest 2011 Jan;139(1):69-79.
Duration:
For the period of hospitalization (UFH/LMWH) or
Extended prophylaxis up to 40 days (Rivaroxaban)
| Surgical patients | |||
|---|---|---|---|
| Risk stratification | Score | Choice of VTE prophylaxis | Remarks |
| Caprini score1 |
<0 At very low risk for VTE |
|
|
|
1–2 At low risk for VTE |
|
||
|
3–4 At moderate risk for VTE |
or
|
||
|
≥5 At high risk for VTE |
|
||
| Duration |
|
LMWH or UFH preferred | |
|
|||
| In case of high bleeding risk |
|
||
VTE: Venous thromboembolism; LMWH: Low molecular weight heparin; UFH: Unfractionated heparin; IVC: Inferior vena cava.
| Orthopedic Surgical Cases | |||
|---|---|---|---|
| Procedure | Duration | Options | Remarks |
| Total hip arthroplasty or TKA | Minimum of 10–14 days |
Any one of LMWH Fondaparinux Apixaban Dabigatran Rivaroxaban, UFH Vit K antagonist Aspirin |
Direct oral anticoagulants (DOACs) are preferred over low-molecular-weight heparin |
| Hip fracture repair | Minimum of 10–14 days |
Any one of LMWH UFH Fondaparinaux Vit K antagonist Aspirin Intermittent pneumatic compression |
|
|
Arthroscopic knee surgery Foot or ankle surgery Upper limb surgery |
6–12 hours after surgery for 14 days (Prophylaxis recommended only if any of the conditions mentioned under Remarks column apply) |
LMWH |
|
TKA: Total Knee Arthroplasty; LMWH: Low molecular weight heparin; UFH: Unfractionated heparin.
Extended thromboprophylaxis is recommended in the outpatient period for up to 35 days from the day of surgery rather than for only 10–14 days.
Dual prophylaxis may be preferred over mono-prophylaxis.
For asymptomatic patients following major orthopedic surgery, Doppler ultrasound screening before hospital discharge is not needed.
| POLYTRAUMA | |
|---|---|
| Category | Recommendation |
| Major trauma and low to moderate risk for bleeding | LMWH or UFH |
| High bleeding risk | Do not use pharmacologic prophylaxis |
LMWH: Low molecular weight heparin; UFH: Unfractionated heparin; VTE: venous thromboembolism.
| ACUTE SPINAL CORD INJURIES | |
|---|---|
| Recommendation | Remarks |
| Mechanical prophylaxis And/Or pharmacological prophylaxis | Consider adding pharmacological VTE prophylaxis with LMWH 24 hours after initial admission if no surgery is planned in the next 24–48 hours, if the benefit of reducing the risk of VTE outweighs the risk of bleeding. |
| Continue VTE prophylaxis in people with spinal injury for 30 days or until the person is mobile or discharged, whichever is sooner | |
VTE: Venous thromboembolism; LMWH: Low molecular weight heparin.
| UROLOGIC SURGERY | |
|---|---|
| Category | Recommendation |
| Transurethral resection of the prostate |
Do not use pharmacologic prophylaxis Instead, consider mechanical prophylaxis |
| Radical prostatectomy | |
| An extended node dissection and/or open radical prostatectomy | May consider LMWH/UFH |
| VTE risk factors | May consider LMWH/UFH |
LMWH: Low molecular weight heparin; UFH: Unfractionated heparin; VTE: venous thromboembolism.
| VASCULAR SURGERY | |
|---|---|
| Category | Recommendation |
| In routine surgeries |
Do not use pharmacologic prophylaxis Instead, consider mechanical prophylaxis |
| Open vascular surgery or major endovascular procedures, including endovascular aneurysm repair, when risk of VTE outweighs risk of bleeding | Consider pharmacological VTE prophylaxis with LMWH for a minimum of 7 days. |
VTE: Venous thromboembolism; LMWH: Low molecular weight heparin.
| LAPAROSCOPIC SURGERY | |
|---|---|
| Category | Recommendation |
| In routine surgeries |
Do not use pharmacological prophylaxis Instead, consider mechanical prophylaxis |
| If risk factors for VTE are present |
Consider pharmacological prophylaxis |
VTE: Venous thromboembolism.
| GYNECOLOGICAL SURGERY | |
|---|---|
| Category | Recommendations |
| Major surgery | LMWH or UFH |
LMWH: Low molecular weight heparin; UFH: Unfractionated heparin.
| NEUROSURGERY | |
|---|---|
| Recommendation | Remarks |
| In routine, mechanical prophylaxis is indicated | Do not use pharmacological prophylaxis routinely. |
| If prolonged immobilization is anticipated, then plan for post-operative pharmacologic prophylaxis | |
| BARIATRIC SURGERY | |
|---|---|
| Recommendation | Remarks |
|
LMWH or UFH or Intermittent pneumatic compression |
Duration: for 10–15 days Dual prophylaxis if risk factors +,
|
OSA: Obstructive sleep apnea; PAH: Pulmonary arterial hypertension; BMI: Body mass index; VTE: Venous thromboembolism; UFH: Unfractionated heparin; LMWH: Low molecular weight heparin.
| MALIGNANCY | ||
|---|---|---|
| Category | Prophylaxis | Remarks |
| Hospitalized cancer patients | LMWH or UFH | Till discharge |
| Cancer patients undergoing surgery |
LMWH or Fondaparinaux or Mechanical prophylaxis (only if the risk of bleed is high) |
Should be prescribed pharmacological prophylaxis in the postoperative period |
| Ambulatory patients (Risk stratification by Khorana score) |
If score < 2 No VTE prophylaxis |
If receiving chemotherapy and at low risk for thrombosis |
|
If score ≥ 2 Rivaroxaban or Apixaban |
Those who are receiving systemic anticancer therapy and are at intermediate-to-high risk of VTE | |
| Multiple myeloma patients |
Aspirin or low-dose Vit K Antagonists or LMWH |
For those receiving lenalidomide, thalidomide, or pomalidomide-based regimens |
| Outpatients with cancer and indwelling central venous catheters |
No role of routine prophylaxis with LMWH or UFH or Vit K Antagonists |
|
VTE: Venous thromboembolism; UFH: Unfractionated heparin; LMWH: Low molecular weight heparin.
|
LONG-DISTANCE TRAVELERS (travel time > 4 hours) |
|
|---|---|
| No risk factors for VTE | High VTE risk |
| Recent surgery, h/o VTE, postpartum, active malignancy, hormone replacement therapy, obesity, or pregnancy | |
| No prophylaxis recommended |
Graduated compression stockings or LMWH or Aspirin |
VTE: Venous thromboembolism; LMWH: Low molecular weight heparin.
| PREGNANCY | |
|---|---|
| Category | Recommendation |
| Women undergoing assisted reproductive therapy |
Routine prophylaxis Not recommended |
| If they develop severe ovarian hyperstimulation syndrome | Prophylactic antithrombotic therapy with aspirin/LMWH recommended |
| Women who have a history of VTE | Postpartum anticoagulant prophylaxis recommended |
| Women with a history of VTE that was unprovoked or associated with a hormonal risk factor | Antepartum anticoagulant prophylaxis recommended |
| Pregnant women having | Family history of VTE | ||
|---|---|---|---|
| None | With family history | Regardless of family history | |
| Antithrombin deficiency | Postpartum prophylaxis | ||
| Antithrombin deficiency or are homozygous for prothrombin mutation |
Antepartum prophylaxis to prevent a first VTE event Not recommended |
||
| Heterozygous for Factor V Leiden or prothrombin mutation |
Postpartum prophylaxis Not recommended |
||
| Heterozygous for Factor V Leiden or prothrombin mutation/protein C or S deficiency |
Antepartum prophylaxis to prevent a first VTE event Not recommended |
||
| Heterozygous for the factor V Leiden mutation or prothrombin mutation or who have antithrombin, protein C, or protein S deficiency |
Postpartum prophylaxis Not recommended |
||
|
protein C, or protein S deficiency, |
Postpartum prophylaxis with LMWH ×6 weeks | ||
| Antithrombin deficiency, and for those who are homozygous for Factor V Leiden mutation or have combined thrombophilias | Antepartum prophylaxis | Antepartum prophylaxis | |
| combined thrombophilias or are homozygous for Factor V Leiden mutation or prothrombin mutation |
Postpartum prophylaxis with LMWH or Vit K antagonists targeted at an INR of 2.0–3.0 for 6 weeks |
||
Note: Wherever not specifically mentioned, no anticoagulation is recommended.
VTE: Venous thromboembolism; LMWH: Low molecular weight heparin; INR: International normalized ratio.
| Level of healthcare | Prophylaxis of VTE by HealthCare Workers | |||||
|---|---|---|---|---|---|---|
| Patient/Family members | ASHA | ANM |
Community Health Officer |
Medical officer | Specialist | |
| Community Level | Follow treatment as prescribed | Advise patients to follow treatment as prescribed | ||||
| Health & Wellness Centre | Advise patients to follow treatment as prescribed | |||||
| Primary Health Centre |
Advise patients to follow treatment as prescribed Counsel on medication compliance |
|||||
|
Community Health Centre |
Prescribe treatment as per guidelines | |||||
| District Hospital | ||||||
|
Medical College Hospital |
||||||
VTE: venous thromboembolism; ASHA: Accredited Social Health Activist; ANM: Auxiliary Nurse Midwife.
ANNEXURE 2
VTE MANAGEMENT: RECOMMENDATIONS
Annexure 2 briefly outlines the task force VTE treatment recommendations through Tables I–XIV and Figure 1 appended below
| Outpatient Treatment (Home based) | ||
|---|---|---|
| In DVT | Uncomplicated DVT and PE at low risk for complications |
Uncomplicated DVT
|
| In PE | HESTIA1 score 0 |
Low-risk PE
|
DVT: Deep vein thrombosis; PE: Pulmonary embolism; RV: Right ventricle; VTE: venous thromboembolism.
| Choice of anticoagulant | |||
|---|---|---|---|
| DOAC | VKA preferred | UFH | LMWH |
| Preferred agent in all settings other than those discussed under alternative agents |
|
|
|
eGFR: estimated glomerular filtration rate; APLA: Antiphospholipid syndrome; DOAC: Direct oral anticoagulants; VKA: Vitamin K antagonists; LMWH: Low molecular weight heparin; PE: Pulmonary embolism; VTE: venous thromboembolism.
| Duration of anticoagulation | ||
|---|---|---|
| Category | Primary phase | Secondary Phase |
| VTE provoked by transient risk factors | 3 months | |
| VTE provoked by chronic (persistent) risk factors | 3 months | Indefinite anticoagulation with periodic assessment (annual) for risk factors of bleeding and risk factors for VTE |
| Unprovoked VTE | 3 months | Indefinite anticoagulation with periodic assessment for risk factors of bleeding |
| HAS-BLED score ≥ 4 | Avoid indefinite anticoagulation | |
VTE: Venous thromboembolism.
Remarks
-
Secondary treatment phase
Vit K Antagonist @ INR 2-3 (A)
Low-dose DOAC (Apixaban/Rivaroxaban) is as good as standard dose DOAC
Aspirin 75 mg or 150 mg daily in people who decline extended anticoagulation treatment or patients with an unprovoked proximal DVT or PE who are stopping anticoagulant therapy.
Routine use of prognostic scores HERDOO2, Vienna and DASH scores, D Dimer testing, or ultrasound to detect residual vein thrombosis is no longer recommended in unprovoked VTE.
APPROACH TO MANAGEMENT
Annexure 2
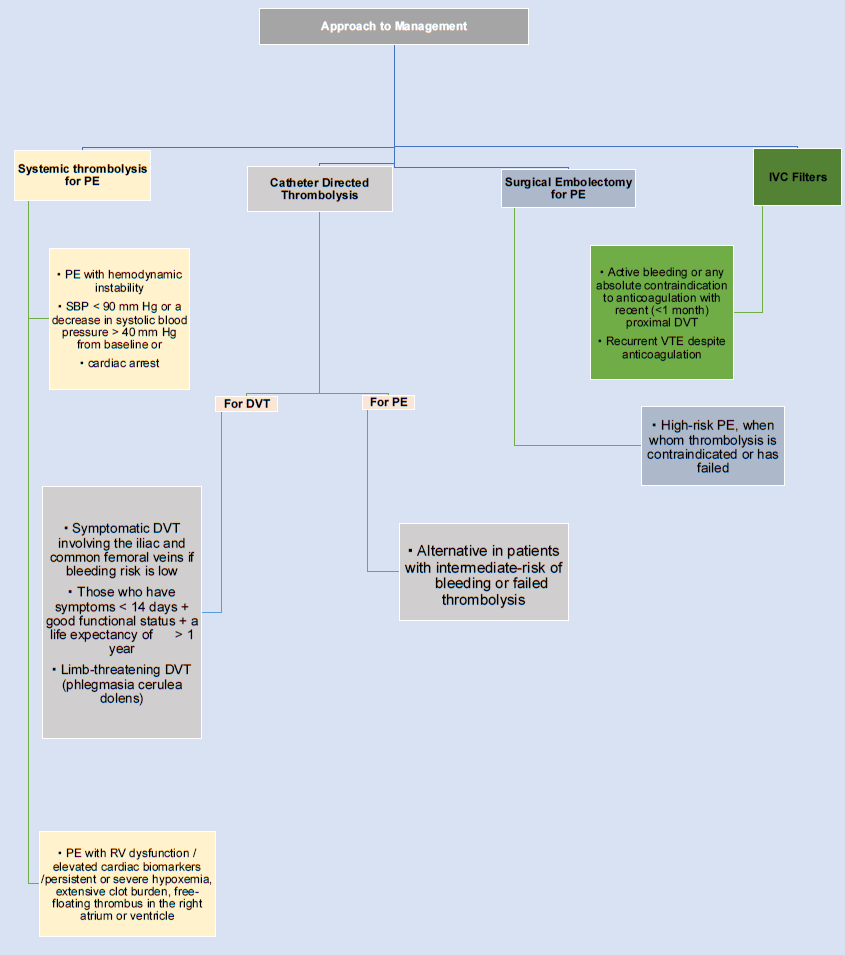
- Approach to management of pulmonary embolism
- DVT: Deep venous thrombosis; IVC: Inferior vena cava; PE: Pulmonary embolism; VTE: Venous thromboembolism; SBP: Systolic blood pressure.
| PREGNANCY | |
|---|---|
| Drug of choice | LMWH over UFH |
| As soon as the patient is in labor, heparin should be stopped immediately | |

|
|
| Spinal/epidural anesthesia or analgesia should be used 12 hours after prophylactic and 24 hours after therapeutic LMWH, while these techniques can be used 6 hours after stopping conventional heparin. | |

|
|
| Heparins should be restarted at least 4–6 hours after removal of an epidural catheter. | |

|
|
| At discharge, the patient may be transitioned to VKAs (safe during breastfeeding). | |

|
|
| Total duration of anticoagulation for pregnancy associated DVT should be at least 3 months of anticoagulants or 6 weeks postpartum whichever is later. | |
| LMWH: Low molecular weight heparin; UFH: Unfractionated heparin; VKAs: Vitamin K antagonist; VTE: venous thromboembolism; DVT: Deep venous thrombosis. | |
| MALIGNANCY | |
|---|---|
| DOAC preferred | Apixaban Rivaroxaban Edoxaban |
| if DOAC cannot be used | LMWH |
| Alternative if patient does not accept injectable therapy | Vitamin K Antagonist |
| Duration |
At least 6 months; Reassess the risk of recurrent VTE and bleeding before deciding on continued anticoagulation. |
DOAC: direct oral anticoagulant; LMWH: Low molecular weight heparin; VTE: Venous thromboembolism.
| CEREBRAL VENOUS THROMBOSIS | ||
|---|---|---|
| Initial phase | Primary & secondary phase | Duration |
| LMWH or UFH | Warfarin or DOAC | 3 months if transient risk factors |
| At least 6 months if unprovoked | ||
| Indefinite if persistent hypercoagulability identified | ||
DOAC: direct oral anticoagulant; LMWH: Low molecular weight heparin; UFH: Unfractionated heparin.
| SPLANCHNIC VEIN THROMBOSIS | |
|---|---|
| Acute SVT | Incidental chronic SVT |
|
Anticoagulation if malignancy or extensive SVT |
SVT: Splanchnic venous thrombosis; DOAC: direct oral anticoagulant; LMWH: Low molecular weight heparin; VKA: Vitamin K antagonists.
|
APLA (Anti Phospholipid Antibody syndrome) |
|
|---|---|
|
Triple positive APLA or Arterial thrombosis or patients with small vessel thrombosis or aPL-related cardiac valvular disease |
Vit K Antagonists preferred over DOAC Indefinite anticoagulation. |
APLA: Anti Phospholipid Antibody syndrome; DOAC: direct oral anticoagulant.
| SUPERFICIAL VEIN THROMBOSIS | |
|---|---|
| Anticoagulation if | Options: |
|
Fondaparinux 2.5 mg SC OD × 45 days Rivaroxaban 10 mg OD or Enoxaparin 40 mg OD × 30 days |
SfVT: Superficial venous thrombosis; SFJ: Saphenofemoral junction; VTE : Venous thromboembolism; SC: subcutaneously; OD: once daily.
| ISOLATED DISTAL DVT | |
|---|---|
| Serial imaging of the deep veins for 2 weeks over anticoagulation |
If severe symptoms or risk factors for extension are absent
|
DVT: deep venous thrombosis; VTE : Venous thromboembolism.
| ISOLATED SUBSEGMENTAL PULMONARY EMBOLISM | |
|---|---|
| Clinical surveillance if low risk for recurrent VTE |
Anticoagulation if high risk for recurrent VTE due to following risk factors:
|
VTE: venous thromboembolism
| Management of HIT | |
|---|---|
| 4T score > 4 | 4T score 4–5 |
| Pretest probability of HIT is intermediate/high | Pretest probability intermediate |
 |
 |
| Discontinue heparin |
Anticoagulation for patients with suspected HIT + Prophylactic dose if bleeding risk is high |
 |
 |
|
Switch to alternate anticoagulant
|
No Vit K Antagonists No prophylactic platelet transfusion |
| Choice of anticoagulant in HIT | |
|---|---|
| Critical illness, high bleeding risk or potential need for urgent procedure. | Argatroban/Bivalirudin |
| Clinically stable | Fondaparinux/DOAC |
| Life or limb threatening thrombosis. | Argatroban/Bivalirudin/Danaparoid Fondaparinux |
| Child-Pugh Class B and C | Avoid DOAC or Argatroban |
 |
|
| Transition to DOAC when patient is clinically stable | |
 |
|
|
Start DOAC
Overlap parenteral agent with warfarin for ≥ 5 days and until INR: 2–3 |
|
| Duration of anticoagulation | 3 months or till platelet recovery if no DVT |
HIT: heparin induced thrombocytopenia; DOAC: direct oral anticoagulants; VKAs: Vitamin K antagonist; INR: International normalized ratio.
| POST THROMBOTIC SYNDROME (PTS) | |
|---|---|
| Prophylaxis | Optimal anticoagulation is key for PTS prevention. |
| Use of compression stockings is not recommended | |
| Catheter directed thrombolysis |
|
| Management |
All cases
|
| Pentoxifylline may be used for treating venous ulcers on its own or with compression stockings. | |
|
Moderate-to-severe PTS Endovascular recanalization or surgical bypass or disobliteration may be considered in patients with chronic venous occlusion class CEAP 4–6 |
|
|
Severely symptomatic patients with PTS Segmental vein valve transfer or venous transposition may be considered |
|
PTS: post thrombotic syndrome; DVT: deep vein thrombosis; CEAP: Clinical Etiological Anatomical Pathophysiological.
| Level of healthcare | Management of VTE by healthcare worker | |||||
|---|---|---|---|---|---|---|
| Patient/family members | ASHA | ANM | Community health officer | Medical officer | Specialist | |
| Community Level | Follow treatment as prescribed | Advise patients to follow treatment as prescribed | ||||
| Health & Wellness Centre | Advise patients to follow treatment as prescribed | |||||
| Primary Health Centre |
Advise patients to follow treatment as prescribed Counsel on medication compliance |
|||||
| Community Health Centre | Advise patients to follow treatment as prescribed | |||||
| District Hospital | ||||||
| Medical College Hospital | ||||||
VTE: venous thromboembolism; ASHA: Accredited Social Health Activist; ANM: Auxiliary Nurse Midwife.
ANNEXURE 3
Annexure 3 describes the various scoring systems recommended by the task force for VTE treatment and prophylaxis through Tables I–IX
SCORING SYSTEMS
| Scoring system | Population at risk | Outputs and risk categories | Lowest risk category | Highest risk category | Comments |
|---|---|---|---|---|---|
| Caprini1 | Surgical patients | Risk of VTE at 3 months |
Lowest risk <0.7% (0 points) |
Highest risk 10.7% (≥9 points) |
No formal validation with original study. External validation studies in surgical subpopulations. |
| Padua Prediction Score2 | Medical inpatients | Risk of VTE at 3 months |
Lowest risk 1.1% (<4 points) |
Highest risk 3.5% (≥4 points) |
Internal validation showing 32-fold variation in VTE risk across 11 studies An external validation in patients with sepsis did not find correlation with VTE risk. |
| IMPROVE Score3 | Medical inpatients | Risk of VTE at 3 months |
Lowest risk 0.4% (0 points) |
Highest risk 5.7% (≥4 points) |
Validation includes 1 retrospective, 1 case control, and 1 prospective multicenter study * |
| Khorana Score4 | Ambulatory cancer patients |
Risk of VTE at 2.5 months |
Lowest risk 0.8% (0 points) |
Highest risk 7.1% (≥3 points) |
Internal development and validation cohort included in original study. Multiple prospective and retrospective validation studies |
Negative predictive value 98.5%, Positive predictive value 6.7%, C-static = 0.7.
VTE: venous thromboembolism.
| PADUA score | |
|---|---|
| VTE risk factor | Points |
| Decreased mobility | 3 |
| Thrombophilia | 3 |
| Previous trauma or surgery within the last month | 2 |
| Age ≥ 70 | 1 |
| Heart or respiratory failure | 1 |
| Ischemic stroke or acute myocardial Infarction | 1 |
| Acute rheumatologic disorder and/or acute infection | 1 |
| Obesity | 1 |
| Hormonal therapy | 1 |
| Score < 4 | Low risk | Confers a <0.3% 90-day risk of symptomatic VTE in patients who do not receive anticoagulation during hospitalization |
| Score ≥ 4 | High risk | Confers an 11% risk of symptomatic VTE |
VTE: Venous thromboembolism.
| IMPROVE VTE score | |
|---|---|
| VTE Risk Factor | VTE risk score |
| Previous VTE | 3 |
| Known thrombophilia | 2 |
| Current lower limb paralysis or paresis | 2 |
| History of cancer | 2 |
| ICU/CCU stay | 1 |
| Complete immobilization ≥1 day | 1 |
| Age ≥ 60 years | 1 |
| IMPROVE: International Medical Prevention Registry on Venous Thromboembolism (VTE); CCU/ICU: Cardiac/Intensive Care Unit. | |
| Score < 3 | Low risk | Confers a <1.5% VTE risk |
| Score ≥ 3 | High risk | Confers an >4% risk of symptomatic VTE |
| 4T Score | |||
|---|---|---|---|
| Category | 2 points | 1 point | 0 point |
| Thrombocytopenia |
>50% fall, or nadir ≥20 × 109/L |
30–50% fall, or nadir 10–19 × 109/L |
< 30% fall, or nadir <10 × 109/L |
| Timing of the decrease in platelet count |
Days 5–10, or ≤ day 1 with recent heparin (past 30 days) |
> Day 10 or timing unclear, or < day 1 if heparin exposure within past 30–100 days |
< Day 4 (no recent heparin) |
| Thrombosis or other sequelae | Proven thrombosis, skin necrosis, or acute systemic reaction after heparin bolus | Progressive, recurrent, or silent thrombosis; erythematous skin lesions | None |
| Other causes of thrombocytopenia | None evident | Possible | Definite |
| Score | Probability | Risk of HIT |
|---|---|---|
| 0–3 | Low | <1% |
| 4–5 | Intermediate | ∼10% |
| 6–8 | High | ∼50% |
Source: Lo GK, et al. J Thromb Haemost 2006 Apr;4(4):759-65.
| IMPROVE BLEED RAM | |
|---|---|
| Risk Factors | Point |
| Moderate renal failure (CrCI 30–50 mL/min) | 1 |
| Male sex | 1 |
| Age 40–84 years | 1.5 |
| Active cancer | 2 |
| Rheumatic disease | 2 |
| Central venous catheters | 2 |
| Admission in intensive care | 2.5 |
| Severe renal failure (CrCI < 30 mL/min.) | 2.5 |
| Liver insufficiency (INR > 1.5) | 2.5 |
| Age ≥ 85 | 3.5 |
| Thrombocytopenia (<50 × 109 cell/L) | 4 |
| Recent (3 months) bleeding | 4 |
| Active gastrointestinal ulcer | 4 |
| High bleeding risk when total score ≥ 7 | 4 |
| Score | Risk | Implication |
|---|---|---|
| <7 | Low | Has a major bleed risk of approximately 0.4% |
| ≥7 | High | Has a major bleed risk of 4.1% |
INR: International normalized ratio; CrCI: Creatinine clearance.
| Caprini score | |||
|---|---|---|---|
| -35 points | 3 points | 2 points | 1 point |
|
|
|
|
| BMI: Body mass index; VTE: venous thromboembolism. | |||
| Score < 2 | Low risk |
| Score 3–4 | Moderate risk |
| Score ≥ 5 | High risk |
| Khorana Score | |
|---|---|
| Patients’ characteristics | Risk score |
| Site of cancer | |
| • Very high risk (stomach, pancreas) | 2 |
| • High risk (lung, lymphoma, gynecological, bladder, or testicular) | 1 |
| Pre chemotherapy platelet count ≥ 350 × 109/L | 1 |
| Pre chemotherapy hemoglobin level < 100 g/L or use of red cell growth factors | 1 |
| Pre chemotherapy leukocyte count > 11 × 109/L | 1 |
| Body Mass Index ≥ 35 kg/m2 | 1 |
| Score 0 | Low risk |
| Score 1–2 | Intermediate risk |
| Score > 2 | High risk |
VTE: venous thromboembolism.
| HESTIA Criteria |
|---|
| If any of the below are answered “Yes,” the patient should NOT be treated as an outpatient. |
| 1. Hemodynamically unstable? |
| 2. Thrombolysis or embolectomy necessary? |
| 3. Active bleeding or high risk of bleeding? |
| 4. Oxygen supply to maintain oxygen > 90% > 24 hours? |
| 5. Pulmonary embolism diagnosed during anticoagulant treatment? |
| 6. In severe pain needing IV pain medication > 24 hours (or multiple doses in the ED)? |
| 7. Medical or social reason for treatment in hospital > 24 hours? |
| 8. Creatinine clearance less than 30 mL/min? |
| 9. Severe liver impairment or disease? |
| 10. Pregnant? |
| 11. Documented history of heparin-induced thrombocytopenia? |
| HAS-BLED-Score | |
|---|---|
| Risk-factor | Scores |
| Hypertension | 1 |
| Abnormal-renal/liver function | 1 or 2 |
| Strokes | 1 |
| Bleeding tendency | 1 |
| Labile-INR | 1 |
| Age (e.g., >65) | 1 |
| Drugs-(e.g., concomitant aspirin, NSAIDs,) or alcohol | 1 or 2 |
| Maximum-score | 9 |
|
Notes: Hypertension is defined as a systolic blood pressure >160 mmHg. 1 point is awarded for each of abnormal renal or liver function, and drugs or alcohol. INR: International normalized ratio; NSAID: Nonsteroidal anti-inflammatory drugs. |
|
| Score | Risk of Bleeding |
| 0–2 | Low risk |
| Score ≥ 3 | High risk |
ANNEXURE 4
DIAGNOSIS OF VTE
Ineffectively managed lower extremity DVT has risks associated with it (e.g., pulmonary emboli) as also the inherent risks of anticoagulation (e.g., resultant major or life-threatening bleeding); hence, the accurate diagnosis of VTE is essential [Figure 1]. Features of lower extremity DVT are usually nonspecific, and many patients are asymptomatic.
History: DVT should be suspected in patients who present with leg swelling, pain, warmth, and erythema. Information that should be sought from patients and informants include:
History of immobilization or (prolonged) hospitalization
Recent surgery or trauma (typically within 12 weeks of surgery or trauma)
Obesity
Previous VTE
Malignancy or symptoms suggestive of malignancy
Use of oral contraceptives or hormone replacement therapy
Pregnancy or postpartum status
Stroke with hemiplegia or immobility
Age > 65 years
Family history of VTE
Heart failure
Inflammatory bowel disease
Physical examination—A thorough physical examination of the legs, abdomen, and pelvis should be performed in patients with suspected DVT to look for the following by the “Look–Touch–Measure” technique:
Dilated superficial veins
Unilateral edema or swelling with a difference in calf or thigh diameters
Unilateral warmth, tenderness, erythema
Pain and tenderness along the course of the involved major veins
Local or general signs of malignancy.
Note: Initial diameters should be recorded at presentation to maintain a baseline. A larger calf diameter is the most useful finding in the presentation. Subsequent measurements are not of much significance, with pain and tenderness being a more reliable indicator. Homans’ sign (calf pain on passive dorsiflexion of the foot) is unreliable for the presence of DVT.
Laboratory Routine laboratory tests (e.g., complete blood count, routine biochemistry tests, liver function tests, coagulation studies) are not useful diagnostically but may provide clues as to the underlying cause and may influence treatment decisions if DVT is confirmed.
Young patients (<40 years) with an episode of unprovoked VTE in unusual sites (cerebral venous sinuses and splanchnic circulation) and those with a history of VTE in the family or recurrent VTE will warrant a screen for inherited thrombophilia. The duration of anticoagulation varies from long term in recurrent VTE to short term (3 months) in the presence of reversible risk factors like surgery. All non genetic tests for thrombophilia (PC, PS, AT, APC-R) should be done after withdrawal of anticoagulation for a period of 4 weeks. However, genetic tests such as FVL, prothrombin gene mutation, and Methylenetetrahydrofolate reductase (MTHFR) gene mutations can be tested during anticoagulation.
In the Indian population, most of the studies have been limited to certain variants only. Many Indian studies have reported the role of MTHFR polymorphisms in VTE risk; however, a recently published report suggests MTHFR polymorphisms should not be a part of inherited thrombophilia testing and eliminating MTHFR from thrombophilia testing will reduce patient concerns and decrease healthcare costs.
Suspected first DVT (risk stratification)
An approach that incorporates clinical assessment of the pretest probability (PTP) and D-dimer testing in selected patients is recommended. This approach allows for the strategic use of US for diagnosis or alternative imaging modalities such as CT or MRI. The goal of diagnostic testing is to “rule-in” (>85% posttest probability of DVT) or “rule out” DVT (<2 % posttest probability of VTE in the next 3 months) with an acceptable level of certainty, thereby justifying instituting or withholding anticoagulant therapy, respectively.
Assessment of clinical PTP In patients with suspected first DVT, we recommend estimation of the clinical PTP. Subsequent measurement of the D-dimer level and compression US are dependent upon the assigned PTP of DVT. We may use the Wells score for the purpose of estimating PTP. Some experts, due to reproducibility, prefer the revised Geneva system as it also overcomes the interobserver variability.
| Wells Score | |
|---|---|
| Clinical feature | Score |
|
Active cancer (treatment ongoing or within previous 6 months or palliative care) |
1 |
| Paralysis, paresis, or recent plaster immobilization of the lower extremities | 1 |
| Recently bedridden for >3 days or major surgery, within 4 weeks | 1 |
| Localized tenderness along the distribution of deep venous system | 1 |
| Entire leg swollen | 1 |
| Calf swelling by more than 3 cm when compared to the asymptomatic leg (measured below tibial tuberosity) | 1 |
| Pitting edema (greater in the symptomatic leg) | 1 |
| Collateral superficial veins (non varicose) | 1 |
| Alternative diagnosis as likely or more likely than that of DVT | –2 |
| Score | Classification |
|---|---|
| 3–8 | High probability |
| 1–2 | Moderate probability |
| ≤0 | Low probability |
| Items | Points |
|---|---|
| Previous PE or DVT | 1 |
| Heart Rate | |
| 75–94 BPM | 1 |
| ≥95 BPM | 2 |
| Previous surgery or fracture | 1 |
| Hemoptysis | 1 |
| Active cancer | 1 |
| Unilateral leg pain | 1 |
| Pain on lower limb palpation and unilateral edema | 1 |
| Age > 65 Years | 1 |
| PE: Pulmonary embolism; DVT: Deep vein thrombosis; BPM: Beats per minute. | |
| 3 Point Score | Clinical Probability |
|---|---|
| ≥5 | High |
| 2–4 | Intermediate |
| 0–1 | Low |
| 2 Point Score | Clinical Probability |
|---|---|
| ≥3 | PE Likely |
| 0–2 | PE Unlikely |
Source: Le Gal G, et al. Ann Intern Med 2006 Feb 7;144(3):165-71.
In patients with suspected first DVT, it is recommended that based on the clinical PTP, further action should be taken:
Low probability—In patients with a low PTP for DVT, we recommend that D-dimer levels be obtained. Patients in whom the D-dimer level is normal (e.g., <500 ng/mL) do not need further testing, while those in whom the D-dimer is positive (e.g., ≥500 ng/mL) should have US of the lower extremities. Patients can proceed directly to US if the D-dimer is expected to be positive due to another condition. DVT is diagnosed if US is positive; no further testing is required if US is negative.
Moderate probability—In patients with moderate PTP for DVT, we recommend that D-dimer levels be obtained. Patients in whom the D-dimer level is normal do not need further testing, while those in whom the D-dimer is positive should have US of the lower extremities. Patients can proceed directly to US if the D-dimer is expected to be positive due to another condition. DVT is diagnosed if US is positive. When neither proximal nor distal DVT is identified on the whole leg US, no further testing is required; in contrast, in those in whom the proximal US is negative, repeat proximal US should be performed at one week to detect extension of distal DVT into the proximal veins.
High probability—For patients with a high PTP for DVT, we suggest that US be performed. DVT is diagnosed if US is positive. If DVT is not identified, options include high sensitivity D-dimer level measurement (if not expected to be positive due to another condition), repeat proximal compression ultrasonography (CUS) at one week (off anticoagulation), whole leg US (if not already performed), or iliac vein US (when iliac vein DVT is suspected). Choosing among these options should be individualized. In general, if one or more of these tests are negative in a patient without proximal DVT on ultrasound, then no further testing is required.
D-dimer
D-Dimer is a degradation product of cross-linked fibrin and is elevated in nearly all patients with acute DVT. However, it is nonspecific since elevated levels are found in many other conditions (e.g., malignancy, sepsis, recent surgery or trauma, pregnancy, renal failure), i.e., D-dimer has high sensitivity but poor specificity for VTE. Hence a negative result (e.g., <500 ng/mL) is useful for ruling out DVT, particularly in those with a low or moderate PTP for thrombosis; however, a negative test is obtained in about 30% of outpatients (lower in inpatients or if there has been a previous VTE). A positive result (e.g., ≥500 ng/mL) is not diagnostic and indicates the need for further investigation. D-dimer testing is of limited value in patients with high PTP since the negative predictive value is lower in this population. In summary, D-dimer assay should not be used as a stand-alone test in patients suspected of having DVT but rather should be used in conjunction with clinical PTP and/or US.
Imaging
CUS with Doppler is the diagnostic test of choice in patients with suspected DVT. In general, the sensitivity and specificity of proximal CUS is greater than 95%. Duplex US has less accuracy than CUS since the specificity of an abnormal duplex ultrasonogram is lower than that of an abnormal compression ultrasonogram. Point-of-care-US is not recommended for diagnosis unless the situation is urgent or emergent.
Ultrasonography interpretation—Interpretation of CUS in patients with a first suspected DVT:
Positive
Using ultrasound probe pressure, the presence of thrombus is diagnosed by demonstrating the noncompressibility of the imaged vein. Veins that can be assessed for compressibility are proximal (e.g., the common femoral, femoral, and popliteal veins) and distal veins (e.g., peroneal, posterior and anterior tibial, and muscular veins); iliac veins often cannot be assessed for compressibility.
Lack of compressibility of a vein with the ultrasound probe is the most sensitive (>95%) and specific (>95%) sonographic sign for proximal vein thrombosis.
The addition of color flow Doppler does not improve the sensitivity but can provide supportive evidence of thrombus and also help to identify calf veins.
Variation of venous size with the Valsalva maneuver has a low sensitivity and specificity for the diagnosis.
In contrast, CUS is less sensitive for the detection of calf vein and iliac vein thrombus since these veins are less readily compressed (particularly calf veins).
Negative—A negative study is one that demonstrates full compressibility of all imaged veins.
Nondiagnostic—A nondiagnostic study is one where there is uncertainty about whether DVT is present or absent.
Nondiagnostic findings are less common in outpatients compared with inpatients, with less than 5% of outpatients expected to have nondiagnostic findings of the proximal veins.
Nondiagnostic findings are also less common when imaging the proximal veins compared with the distal veins (i.e., with whole leg US); however, nondiagnostic findings that are confined to the distal veins are also less important and can usually be managed by withholding anticoagulant therapy while doing serial ultrasound testing.
The main reasons for a nondiagnostic examination are:
-
(aa)
difficulty visualizing the deep veins because of morbid obesity, edema, recent surgery or trauma, skin lesions, contractures, or leg casts.
-
(ab)
although the deep veins are well visualized, small or atypical appearing abnormalities of uncertain significance may be identified.
-
(ac)
in patients with previous DVT, when a thrombus is present, it is often difficult to assess if it is acute or old (residual thrombosis can persist indefinitely).
-
Further investigation(s) (e.g., repeat proximal CUS at three and seven days) in those with nondiagnostic studies should be individualized and depend upon why the US is considered nondiagnostic, the extent and position of the venous segment (e.g., distal or proximal veins) that is nondiagnostic, clinical PTP, results of D-dimer testing, and the clinician’s overall assessment of the risk associated with undiagnosed DVT.
Imaging at first occurrence
For patients who are not initially stratified according to clinical PTP of low, moderate, or high risk, the initial test of choice is US.
When the whole leg US is negative, no further investigations are necessary unless iliac vein thrombosis is suspected.
When the proximal CUS is negative, options include whole leg US (to detect distal DVT), repeat proximal CUS at 3–7 days (to detect extension of distal DVT into the proximal veins), measuring a high-sensitivity D-dimer level, or assessing clinical PTP (low PTP excludes DVT with a negative proximal CUS).
Diagnostic compressive US
Proximal CUS—In most patients with suspected DVT, CUS with Doppler is the imaging test of choice. The presence of DVT is diagnosed by demonstrating noncompressibility of the imaged vein.
Whole leg CUS—Both proximal and whole leg US have a high sensitivity for the detection of thrombus in the proximal veins (i.e., common femoral, femoral, and popliteal veins). Whole leg US additionally examines the veins in the calf (peroneal, posterior and anterior tibial, and muscular veins) and can, therefore detect isolated distal DVT.
Imaging in Recurrence
For most patients with suspected ipsilateral DVT recurrence, proceeding directly to US (proximal or whole leg US) or using an approach similar to that described for the first suspected DVT is appropriate. When an ultrasonographic abnormality is identified in patients with suspected recurrence, it may be difficult for the clinician to determine whether it is due to an old or new thrombus. The availability of a previous ultrasound report that documents the extent of residual thrombosis greatly improves the accuracy of ultrasound for recurrent DVT. In the absence of a previous ultrasound report, magnetic resonance direct thrombus imaging (MRDTI) may be useful.
Alternative imaging modalities—For patients with suspected DVT, contrast-enhanced computed tomographic venography (CTV) and magnetic resonance venography (MRV) are rarely used diagnostically, unless there is uncertainty about iliac vein or IVC thrombosis after US. Ascending contrast venography, which was the earlier gold standard for DVT diagnosis, and impedance plethysmography are now not recommended to be used.
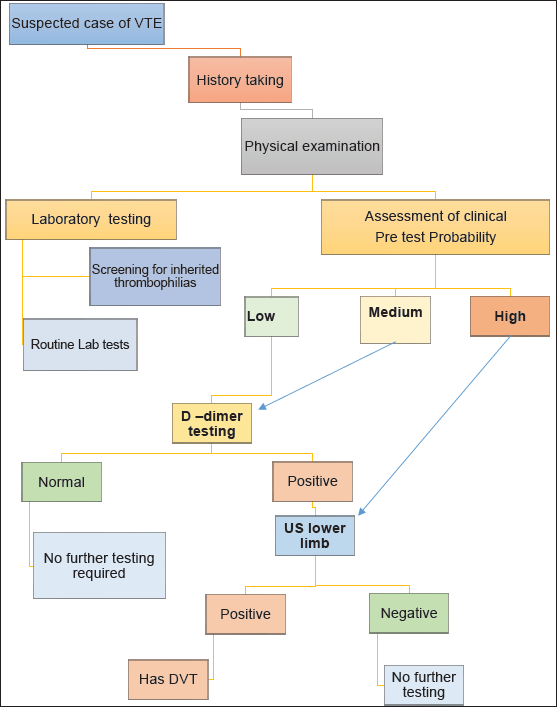
Clinical Decision Aid – Diagnostic workup of a suspected case of VTE
DVT: Deep venous thrombosis; VTE: Venous thromboembolism; US: Ultrasonography.
ANNEXURE 5
MOLECULAR ASPECTS AND GENETIC BACKDROP OF VTE
Ordering thrombophilia tests is easy; determining whom to test and how to use the results is not (Connors 2017).
To understand the Indian perspective of genetic risk factors in VTE, a literature review was undertaken to identify the genetic variants that have been reported in association with VTE [Table I].
Indian data depict that the established thrombophilia genetic markers FV Leiden and Prothrombin G20210A have a limited role from the Indian subcontinental perspective. Several studies have shown the role of FV Leiden in VTE risk, however, only with certain comorbidities in the Indian population. To understand this limited role, we reviewed the literature from other Asian countries also. The data show no role of established genetic markers in the Chinese and Thai populations as well. The prevalence of the FV Leiden phenotype, shown by Ridker et al. is 0.4% in an Asian population.
In the Indian population, most of the hospital-based studies have been limited to certain variants only. Many studies have reported the role of MTHFR polymorphisms in VTE risk; however, a recently published report suggests MTHFR polymorphisms should not be a part of inherited thrombophilia testing and eliminating MTHFR from thrombophilia testing will reduce patient concerns and healthcare costs.
| Variant | Association | Sample Size | Author & Journal |
|---|---|---|---|
| Factor V Leiden G1691A mutation and prothrombin G20210A | Significantly associated in the Kashmiri population |
250 patients 250 controls |
Shafia et al., Gene (2018) |
| MTHFR C677T polymorphism | No significant association in the Kashmiri population | ||
| EDN T1370G (endothelin gene) | Significant association with VTE occurrence | 133 patients with VTE 164 controls | Kumari et al., Clinical and Applied Thrombosis/Hemostasis (2017) |
| CYP4F2 1347 G> A polymorphism | Significant association with PVT (portal vein thrombosis) |
91 PVT cases 136 controls |
Kalpana et al., Medicine (2019) |
| JAK2V617F mutation | May increase the risk of thrombosis in patients with Philadelphia negative chronic myeloproliferative neoplasms. | 65 (46 males and 19 females) CMPN cases | Singh et al., Indian J Pathol Microbiol (2018) |
| FV Leiden | Inherited APCR in patients with DVT—significant association |
50 APCR + patients 50 controls |
Sharma et al., Clinical and Applied Thrombosis/Hemostasis (2017) |
| Factor V leiden (FVL) mutation and PAI 4G/4G homozygosity | Increase DVT risk in pregnant women in Western India | Prevalence of DVT in 34,720 prenatal women | Vora et al. Thrombosis (2007) |
| Factor V Leiden | Significant Association with Myocardial Infarction Patients |
120 patients of MI (age < 40) 100 controls |
Khare et al., Indian Journal Of Medical Sciences (2004) |
| MTHFR 677TT polymorphism | Increased risk of thrombosis in patients with hyperhomocysteinemia | 124 patients with DVT | Paradkar et al., Indian Journal of Clinical Biochemistry (2020) |
| MTHFR 677C/T | Contribute toward susceptibility to thrombosis |
93 male patients 102 controls |
Kumari et al., Thrombosis (2014) |
| PAI-1 −844G/A, fibrinogen-β −455G/A | Protective role | ||
| FVL (1691G/A), pro-thrombin (20210G/A), and TFPI (−536C/T) | Limited role in Indian population | ||
| eNOS894G/T and 2479G/A polymorphisms | Possess the risk of VTE |
100 cases of DVT 200 controls |
Akhteret al., Clinical Laboratory (2022) |
| MTHFR C677T and prothrombin G20210A mutation | No significant association in west Indian population |
Cases of DVT 252 males 180 females |
Ghosh et al., Clinical and Applied Thrombosis/Hemostasis (2001) |
| Variant allele 4G of PAI-1 4G/5G polymorphism | Significantly associated with ischemic stroke in young Indians |
100 cases 100 controls |
Akhter et al., Clinical and Applied Thrombosis/Hemostasis (2017) |
| TFPI polymorphisms (polymorphisms (33T > C, 399C > T, and 536C > T)) | 33T > C protective and 399C > T as risk factors |
100 DVT patients 100 controls |
Kamal et al., Blood Cells, Molecules, and Diseases (2017) |
| CYP2C9 polymorphisms (rs1799853, rs1057910, rs1057909, and rs28371686), VKORC1 promoter polymorphism (rs9923231) | CYP2C9 and VKORC1 polymorphisms, suggested that an increase in the anti-coagulant drug dose may be necessary for Indian patients | 124 patients with DVT | Arunkumar et al., Drug Discoveries & Therapeutics (2017) |
VTE: venous thromboembolism; PVT: Portal vein thrombosis; DVT: Deep vein thrombosis; MI: Myocardial infarction; APCR: Activated protein C Resistance; CMPN: Chronic Myeloproliferative Neoplasms.

- Algorithm for Selecting Patients with a First VTE episode for Thrombophilia Testing.
- VTE: venous thromboembolism; FVL: Factor V leiden; PTG: Prothrombin gene mutation; MPN: myeloproliferative neoplasms; PNH: Paroxysmal Nocturnal Hemoglobinuria; aPL: Antiphospholipid antibody.
We also reviewed the literature regarding European and American populations and a recently published trans-ancestry meta-analysis, and also another independent study that tested approximately 13 million DNA sequence variants for association with VTE and reported the variants associated with VTE [Figure 1]. A similar approach could be replicated in the Indian population, and validation of these variants in the context of different regional/ethnic population groups could be considered to identify a set of variants uniquely associated with Indian population (if at all).
Hence, a large-scale population-based genome-wide association study is recommended amongst the Indian population with representation from different regional and ethnic groups to identify the genetic associations with VTE.
ANNEXURE 6
PUBLIC HEALTH RESPONSE TO REDUCING DVT AND PE
DVT and PE are major public health problems across the world today. It is essential for policymakers to direct public health responses to reducing the burden of VTE, in various settings. The required set of actions is ideally organized by the CARE framework to be customized to the requirement and context on a regional basis:
Communication refers to the provision of information to motivate and empower individuals and healthcare professionals in various settings to catalyze change that will lead to more effective prevention, diagnosis, and treatment of first-time and recurrent DVT/PE.
Action refers to interventions and activities that will assist various stakeholders in preventing, screening, diagnosing, and managing medical conditions or diseases more effectively.
Research into the various aspects of VTE that may be required to be addressed or investigated further.
Evaluation refers to the ongoing assessment of the various activities and interventions for VTE to ascertain their practicality, feasibility, cost benefit, etc.
Note: The US Surgeon General’s “call to action to prevent DVT and PE” (2008) provides an excellent framework which can be suitably adapted to the Indian context.
Setting: Communities
The community, which comprises individuals and their families, needs to understand DVT and PE as threats to their health and life; they need to understand the risk factors for these conditions and to learn how to reduce these risks. They need to recognize the signs and symptoms and to know about available medical management modalities. Patients and their family members should actively discuss these conditions when interacting with their healthcare providers. The goal is to raise awareness among patients and their family members and empower them to ask their healthcare providers about preventive treatment during hospitalization, after a traumatic event, or in other high-risk situations.
A broad-based health risk communication campaign can play a major role in raising awareness at the community level. From a public education and social marketing standpoint, communication campaigns can disseminate structured health messages aimed at educating individuals about DVT/PE. It is essential to harness social and other media to address gaps that exist in the availability of appropriate educational materials pertaining to VTE, among other medical conditions. Emphasis should be placed on opportunities for communication at the family and community level, with a specific focus on high-risk groups.
Communication
Policymakers and healthcare administrators need to take the following steps, among others:
Increasing public knowledge of DVT/PE and the severity of the burden these disorders place on society.
Educating people about the signs, danger signs, and precipitating circumstances, including medical procedures, hospital stays, and trauma.
Educating the general public on genetic predispositions to DVT/PE.
Supporting public education by letting people know how serious the issue is in terms of incidence and mortality/morbidity.
Publication of specific patient tales via news channels and social media, as human interest stories frequently have a greater impact than statistics alone.
Highlighting the substantial gap between what is understood about how to prevent and treat DVT/PE and what is happening in practice today.
Facilitating the development and dissemination of uniform messages about DVT/PE that are consistent with existing guidelines.
Action
Actions that need to be initiated by policymakers and medical administrators include but are not limited to:
Forming community coalitions to sponsor public awareness campaigns.
Developing tools and materials that patients can use when talking with their physicians and other health professionals.
Creating local networks and peer support programs for patients and their family members.
Working with volunteer groups, professional societies, and the media as part of a national awareness campaign intended to educate both the public and health professionals about the incidence of the disease, along with its symptoms and risk factors.
Making available to the media accurate messages about DVT/PE for news stories and media programming, including television shows or podcasts.
Encouraging community-based advertising campaigns.
Consider using a celebrity spokesperson to deliver messages about these conditions, especially a celebrity who may have had a personal experience related to either DVT or PE.
Research and Evaluation
It is recommended for policymakers and healthcare system administrators to assign priority to this often neglected aspect of interventions focused on medical conditions. This is essential to:
Gain a better understanding of what the public already knows about DVT/PE, gaps in their understanding, and how best to address those gaps.
Develop and test messages to determine which approaches work best to educate the public, inform them of when they are at risk, and empower them to raise issues proactively with their clinicians.
Investigate, in a culturally and linguistically appropriate manner, why certain ethnic groups are more or less likely to develop these conditions.
Investigate the causes of age- and gender-based variations in the incidence and recurrence of these diseases, including why men are more susceptible to a recurrence.
Research the role (if any) that behavior modification (e.g., smoking cessation, increased physical activity, weight loss) plays in reducing risk.
Conduct research to better understand why obesity increases risk.
Investigate the role that prolonged immobility due to travel (air, car, rail), hospitalization, or confinement to bed plays in increasing risk.
Conduct an analysis of the economic toll of DVT/PE on individuals, families, communities, and the nation as a whole. This analysis should include not only the direct costs (i.e., healthcare expenditures) but also indirect costs such as lost productivity and wages due to time away from work.
Evaluate the impact of communication and social marketing programs, including pre- and post-evaluation levels of consumer awareness and knowledge.
Conduct formative research to ensure that media messages are positive, realistic, relevant, consistent, and effective.
Setting: The Healthcare System
The healthcare system is uniquely positioned to implement interventions aimed at reducing the incidence and burden of DVT/PE, as the majority of cases occur within the healthcare system itself. There is a gap between the implementation of a required standard of care with broad compliance to evidence-based guidelines and ground realities.
Healthcare systems in the public and private sectors and also medical colleges have a crucial role to play in preventing and reducing the burden of DVT/PE. Much is left to be done—to apply evidence-based medicine in real-world settings and to investigate the gaps in knowledge related to the management of VTE.
Communication
Actions that need to be initiated by policymakers and healthcare administrators include but are not limited to:
Informing healthcare professionals and administrators about the problem of DVT/PE in terms of mortality, morbidity, and direct and indirect costs.
Promoting evidence-based practice by sharing existing guidelines with healthcare professionals on the prevention, diagnosis, and treatment in specific at-risk populations.
Promoting the findings and recommendations from expert groups such as professional societies related to the importance of screening all hospitalized patients for risk for these diseases and providing appropriate preventive treatment based on those screenings.
Educating healthcare professionals about the availability of genetic testing wherever feasible, about when it may be appropriate to discuss with and test patients, and the importance of counseling for those who test positive.
Educating healthcare professionals at all levels about the relative risks of excessive bleeding from properly managed anticoagulation therapy versus the risks of not using such therapy.
Informing healthcare professionals about the availability and appropriate use of treatment options, including anticoagulation therapy and clot dissolving/clot removal therapies.
Action
Actions that need to be initiated by policymakers and healthcare administrators include but are not limited to:
Convening a TF, such as the NAMS TF, to forge consensus on a single set of clear, standardized, evidence-based guidelines in those areas where multiple and/or conflicting guidelines currently exist.
Instituting formal systems related to risk assessment and the provision of preventive therapy (prophylaxis) to appropriate high-risk individuals in the healthcare system and community.
Consistently tracking performance on current and future DVT measures that are endorsed by professional societies and developing quality improvement initiatives designed to improve performance on these measures over time.
Developing and improving easy-to-use tools (such as Internet-based apps) that provide ready access to relevant data and information at the point of care. These tools will help healthcare professionals to follow existing evidence-based guidelines and assist in enhancing their patient care service delivery.
Developing and/or refining tools and/or algorithms to determine who should undergo diagnostic imaging tests for DVT/PE. These tools could incorporate clinical manifestations, biomarkers and genetic profiles, patient and family history, the results of simple tests, and other information to determine who should be screened.
Identifying and supporting healthcare professionals who can provide evidence-based preventive, diagnostic, and therapeutic care and serve as models in their respective hospitals.
Encouraging medical and nursing colleges to provide adequate education and training to ensure that the new generations of doctors and nurses are aware of the magnitude of the problem and how to prevent, diagnose, and treat DVT/PE in accordance with the latest scientific evidence.
Encouraging medical and nursing colleges, and other organizations to incorporate training into continuing medical education and recertification processes such as renewal of registrations.
Supporting the development of hospital- and community-based support programs for patients with DVT/PE and their family members.
Research and Evaluation
It is recommended for policymakers and healthcare system administrators to assign priority to this often neglected aspect of interventions focused on medical conditions. This is essential to:
Conduct further research into the benefits and risks associated with various strategies (pharmacological, mechanical, and surgical) for dissolving or removing clots and for determining which patients, if any, would benefit from these approaches (as an alternative to anticoagulation therapy).
Conduct further research into the pathophysiology of DVT/PE, including the roles of inflammation, obesity, stasis, and the basic endothelial cell biology and vessel response to stasis and thrombosis. This research can lead to the development of novel prevention and treatment strategies.
Investigate whether more biomarkers can be identified that will allow for the development of individualized risk profiles for primary and recurrent DVT/PE and chronic venous insufficiency. These biomarkers can possibly be used to help predict an individual’s response to therapy.
Investigate the role of prolonged air, car, boat, or rail trips (and other situations causing long periods of immobility) on raising risk, both for the general population and certain high-risk groups, such as women on oral contraceptives or individuals with a genetic predisposition to DVT/PE.
Investigate the role of CUS in diagnosing isolated calf DVT, and study the benefits and costs associated with treatment.
Continue to study the effectiveness of various tests, including the D-dimer and other tests, in diagnosing the recurrence of the disease.
Investigate the safety and effectiveness of various approaches to diagnosing DVT/PE in pregnant women.
Conduct further research into the best drugs, dosing strategies, and treatment regimens for anticoagulation therapy for certain patient populations, including children (from infancy through adolescence), obese individuals, and those with renal insufficiency.
Conduct further research on the benefits and risks of preventive and therapeutic anticoagulation therapy for certain patient populations, including children, pregnant women, individuals with a genetic predisposition to DVT/PE (with or without prior events), cancer patients, and the elderly. Such research should also address how to treat individuals with multiple risk factors, such as pregnant women or children with genetic predisposition.
Conduct further research into the appropriate duration of anticoagulation therapy in specific patient populations, including whether some high-risk groups should remain on the therapy indefinitely.
Investigate the role that pharmacogenetics can play in determining optimal warfarin dosing in individuals.
Investigate and evaluate the various approaches (e.g., pharmacological, mechanical, and/or a combination) to reduce the risk and impact of chronic venous insufficiency.
Conduct research into when genetic testing is appropriate, including whether and when to test the asymptomatic family members of those with a genetic predisposition to DVT/PE.
Conduct further research into optimal therapy for those with genetic predisposition and how that therapy might vary depending upon the number of genetic and other acquired risk factors or triggering events. Research should focus on the impact of specific thrombophilic disorders on anticoagulant therapy management and the identification of optimal prophylactic strategies for asymptomatic individuals during high-risk situations.
Conduct research into how upper extremity DVT—a less common and less studied form than DVT in the legs—should best be evaluated, diagnosed, and managed.
Setting: Policymakers and Governments
Healthcare policymakers at the state and national levels have a crucial role to play in raising awareness and encouraging the development and use of evidence-based guidelines. The scientific community and professional societies, such as the National Academy of Medical Sciences (India), Indian Society of Hematology, Indian Public Health Association, and the Indian Medical Association, must seek to collaborate, partner, facilitate, and steer actions required for advocacy with all stakeholders. Governments at the state and the national level, along with the scientific community, must work together to focus on the following areas:
Communication
Raise policymakers’ awareness of DVT/PE and the magnitude of the problems caused by the disease, as well as the need to support research and infrastructure that are consistent with the provision of evidence-based care.
Support public awareness campaigns.
Support the education of health professionals, including the dissemination of evidence-based guidelines.
Action
Review Ayushman Bharat reimbursement policies to ensure that they encourage the provision of evidence-based prevention, diagnosis, and treatment. Subsequently, to make recommendations to the Government of India for necessary action as may be required.
Support the formation of community-based regional and national multi stakeholder coalitions dedicated to raising awareness about these VTEs.
Form TFs, steering committees, or advisory committees dedicated to addressing the problems resulting from VTE.
Support actions that lead to enhanced awareness about DVT/PE among healthcare professionals and obtain better adherence to evidence-based practices.
Research and Evaluation
Support basic, clinical, and epidemiological research that is intended to fill critical gaps in the current knowledge about DVT/PE.
Support translational research and the development of other tools that are intended to speed the adoption of new scientific knowledge into the everyday practice of medicine.
Support the training of scientific investigators and healthcare professionals who are interested in VTE.
ANNEXURE 7
VTE TRAINING AND EDUCATION FOR HEALTHCARE PROFESSIONALS
It is recommended that an online training course for healthcare professionals be proposed by NAMS to be hosted on the Government of India website for training (www.igot.gov.in). This training course may be updated periodically as per evolving evidence and peer-reviewed best practices.
An outline template for such a training course may be taken from
https://www.cdc.gov/ncbddd/dvt/training.html#accreditation
Stop the Clot®: What Every Healthcare Professional Should Know
Course Overview: A self-paced, online course providing the most current foundational information on assessing, treating, and managing patients who have blood clots and clotting disorders.
Target Audience: Physicians, Nurses, and other healthcare professionals
Content:
Basics of blood clots
Thrombophilia and blood clots
Anticoagulation medications
Post-thrombotic syndrome
Pulmonary hypertension
Prevention of blood clots
Course Objectives
At the conclusion of this proposed training course, participants should be able to do the following:
Explain three signs and symptoms of DVT and pulmonary embolism (PE).
Describe three factors that increase the risk of developing a blood clot in the form of a DVT and/or PE.
Describe three management considerations for the use of anticoagulant medications.
Explain three signs and symptoms of post-thrombotic syndrome that providers should include in patient education plans for blood clot survivors.
Explain two facts about pulmonary hypertension and its relationship to PE.
Describe three appropriate treatment/management options to prevent blood clot recurrence and secondary complications.
ANNEXURE 8

VTE: venous thromboembolism.
ANNEXURE 9
Comprehensive primary healthcare framework approach: prevention of VTE

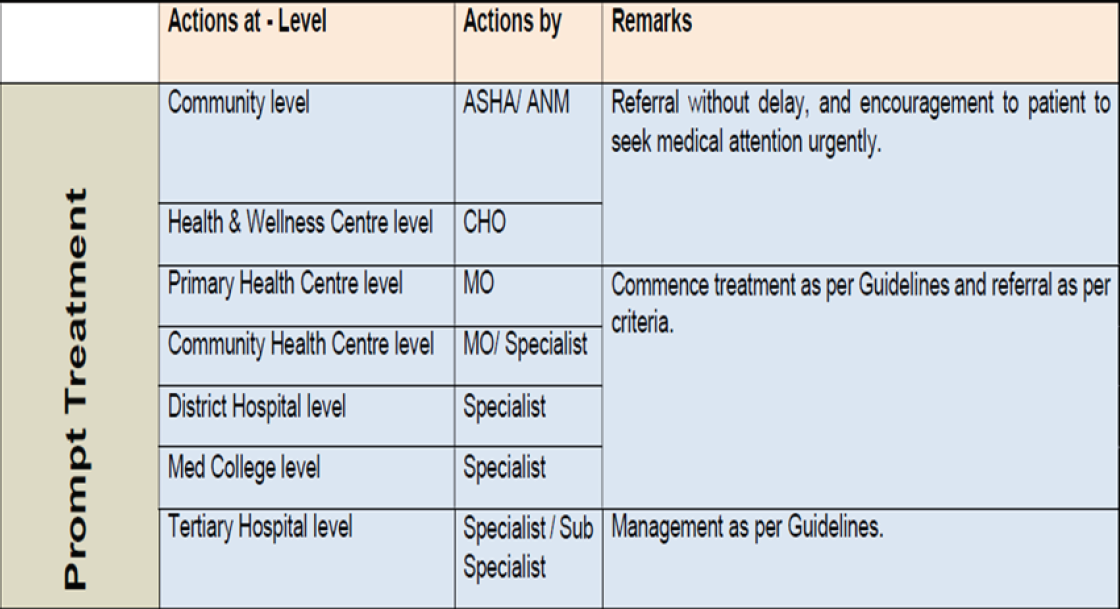
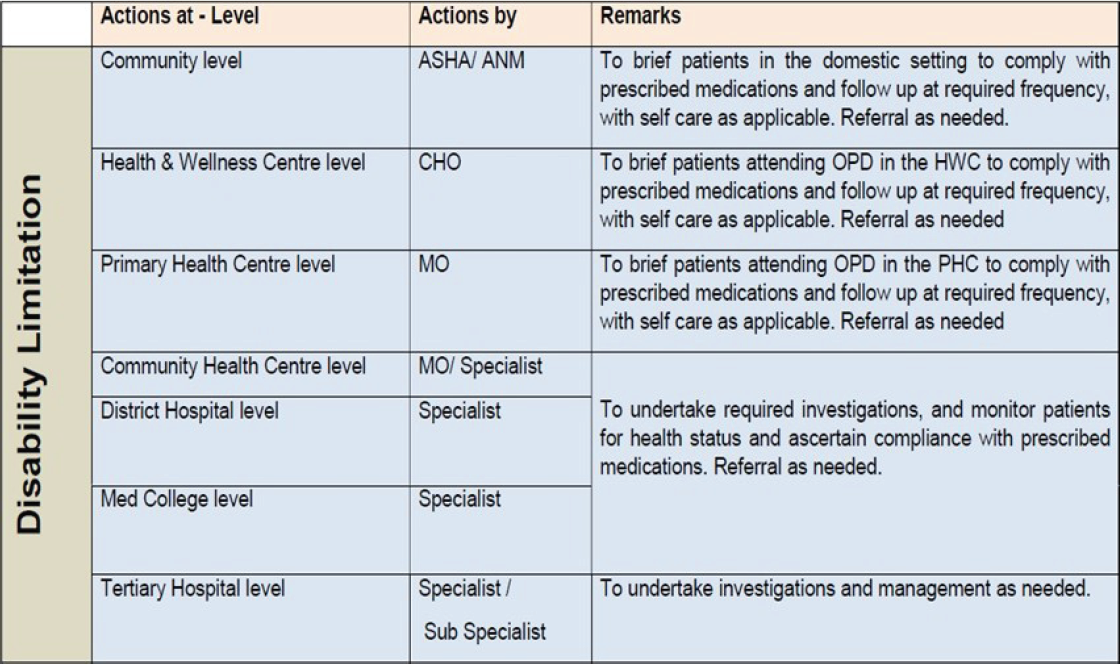
ASHA: Accredited Social Health Activist; ANM: Auxiliary Nurse Midwife; CHO: Community Health officer; MO: Medical Officer.
ANNEXURE 10
Areas of research/future directions
Advances in technology and pharmacology have already improved our ability to predict and prevent VTE. However, the following major barriers to improving VTE prevention still exist:
the limitations on the overall benefit to thromboprophylaxis inherent to current anticoagulant medications.
the imperfect science of individualizing VTE risk stratification and the inherent complexity of predicting multifactorial and competing phenomena.
As per various position papers, future direction can be focused toward therapeutics and technology, including the importance of antithrombotic options with less bleeding and technological advances, including artificial intelligence and machine learning to refine risk stratification and facilitate their implementation.
A more pragmatic approach to pharmacologic prevention is what is necessitated in India today given the diversity in healthcare settings. Over the past decade, the tolerability, acceptability, and quality of life for patients at risk of VTE have changed with the advent of effective oral therapies for VTE treatment and prevention. In those with cancer, patient-reported quality of life is better with oral anticoagulant treatment compared to daily subcutaneous LMWH injection. While not as rigorously studied, quality-of-life improvements with oral therapies are likely similar in the prophylactic setting.
Novel agents for pharmacologic prophylaxis are currently being developed that target various factors. If these trials or other new agents are successful at reducing VTE without substantially increasing the risk of bleeding, existing approaches to VTE prevention could change dramatically.
Efforts to predict and prevent venous thromboembolic disease are predicated on our ability to accurately identify patients at risk. The benefits of thromboprophylaxis must be weighed against the financial costs and potential for increased bleeding. Understanding which patients are at greatest risk can help healthcare professionals and their patients to make informed decisions about the use of anticoagulants to prevent VTE. We must continue to collect and analyze large datasets on patient-specific and acquired risk factors and how they interact to improve existing risk assessment models.
The discovery and evaluation of novel biomarkers to risk-stratify patients may be an area of significant interest going forward, and serum biomarkers such as Vascular Endothelial Growth Factor (VEGF), Interferon-alpha, Interleukin-15 (IL-15), and Citrullinated histone H3 (H3cit) may see further investigation for this purpose.
The development and validation of prediction models should seek to increase the accuracy of prediction without sacrificing usability. As we seek to individualize preventive efforts, the primary obstacles that affect the implementation of risk assessment models will be the complications and complexity of any proposed algorithm. Scoring systems and other risk-assessment models can be implemented for use by healthcare professionals if they are easily accessible and understood, but developing sufficient predictive power for VTE often requires the combination of several variables. Scoring systems can quickly become time-consuming for use by physicians, and hiring additional data entry personnel adds significant cost. Predictive algorithms that appropriately consider and calculate bleeding risks for individual patients to avoid harm from VTE prophylaxis increase complexity and add additional workload. Scoring systems developed in the present times must not lose sight of the practical challenges of implementation by treating clinicians in busy hospital settings.
We have made tremendous progress in understanding the epidemiology and prevention of VTE and have transitioned from studies detailing the benefits of “mini-dose” unfractionated heparin given to postoperative patients to sophisticated algorithms that leverage patient-specific and acquired risk factors to determine which patients will derive the greatest benefit from VTE prophylaxis. As scoring systems and other decision support tools increase in accuracy and complexity, we risk overburdening overworked clinicians, and hence, we need to be aware of this pitfall.
ACKNOWLEDGMENT
We are grateful to the tireless guidance and inspirational leadership provided by Lt Gen Velu Nair, Chairperson of the VTE TF in identifying, perusing and distilling a multitude of international guidelines focused on various aspects of VTE and developing an actionable and up-to-date guidance for the Indian context taking into consideration published Indian data on the subject.
We profusely thank Prof Pankaj Malhotra from PGI Chandigarh; Dr. Soniya Nityanand from RML Institute of Medical Sciences, Lucknow; Dr. Manisha Madkaikar from NIIH Mumbai; and Prof Mohammad Zahid Ashraf, from Jamia Millia Islamia, for their invaluable contribution to the various sections. The co-opted members, Dr. Bipin Kulkarni from NIIH and Dr. V.A. Arun from PGI Chandigarh, ably supported the TF in developing these guidelines.
The TF Secretariat undertook extensive reviews of literature and evidence and collated them with an overarching view to present an easy-to-refer format for clinicians in the Indian context. The public health and health systems focus and orientation was maintained consistently through the proceedings of the TF and the resultant document by the efforts of the Secretary, Col MP Cariappa.
The contributions of various other Hemato-Oncologists and other domain experts are acknowledged.
OPERATIONAL DEFINITION OF TERMS USED
Pulmonary embolus refers to obstruction of the pulmonary artery or one of its branches by material (e.g., thrombus, tumor, air, or fat) that originated elsewhere in the body. Patients with acute PE typically develop symptoms and signs immediately after obstruction of pulmonary vessels. Some patients with PE may also present subacutely within days or weeks following the initial event. Patients with chronic PE slowly develop symptoms of pulmonary hypertension over many years (i.e., chronic thromboembolic pulmonary hypertension; CTEPH).
Symptomatic PE refers to the presence of symptoms that usually leads to the radiologic confirmation of PE, whereas asymptomatic PE refers to the incidental finding of PE on imaging (e.g., contrast-enhanced computed tomography performed for another reason) in a patient without symptoms.
LIST OF ABBREVIATIONS
ACCP: American College of Chest Physicians
AHA: American Heart Association
APLA: Anti-phospholipid antibody syndrome
ASCO: American Society of Clinical Oncology
ASH: American Society of Hematology
BMI: Body mass index
CrCl: Creatinine clearance
DOAC: Direct acting oral anticoagulant
DVT: Deep venous thrombosis
ENDORSE: Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting
ESA: European Society of Anesthesiology
ESC: European Society of Cardiology
HIT: Heparin-induced thrombocytopenia
IPC: Intermittent pneumatic compression
ISHBT: Indian Society of Hematology & Blood Transfusion
ITAC: International Initiative on Thrombosis and Cancer
IVC: Inferior vena cava
LMWH: Low molecular weight heparin
NICE: National Institute of Clinical Excellence
OSA: Obstructive sleep apnea
PAH: Pulmonary artery hypertension
PE: Pulmonary embolism
PICO: Population, intervention, comparator, and outcome
PTS: Post-thrombotic syndrome
RV: Right ventricle
SVT: Splanchnic vein thrombosis
TF: Task Force
UFH: Unfractionated heparin
VKA: Vitamin K antagonist
VTE: Venous thromboembolism
VT: Venous thrombosis
SUGGESTED READINGS
- 16th International Congress on Antiphospholipid Antibodies Task Force. Report on Antiphospholipid Syndrome Treatment Trends: Lupus. 2020;29:1571-93.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Deep vein thrombosis in Indian patients undergoing major lower limb surgery. Indian J Surg. 2003;65:159-62.
- [Google Scholar]
- The Postthrombotic Syndrome- Guidelines. Circulation. 2014;130:1636-61.
- [CrossRef] [PubMed] [Google Scholar]
- 2018 guidelines for management of venous thromboembolism: Venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2:3317-59.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- 2019 guidelines for management of venous thromboembolism: Prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019;3:3898-944.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4:4693-738.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021;5:927-74.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- From Classical laboratory parameters to novel biomarkers for the diagnosis of venous thrombosis. Int J Mol Sci. 2020;21:1920.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antithrombotic Therapy and prevention of thrombosis, evidence-based clinical practice guidelines. Chest. 2012;141:e419S-e494S.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antithrombotic therapy for VTE disease guideline and expert panel report. Chest. 2016;149:315-52.
- [CrossRef] [PubMed] [Google Scholar]
- Antithrombotic therapy for VTE disease: Second update of the CHEST guideline and expert panel report. Chest. 2021;160:2247-59.
- [CrossRef] [PubMed] [Google Scholar]
- From classical laboratory parameters to novel biomarkers for the diagnosis of venous thrombosis. Int J Mol Sci. 2020;21:1920.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Incidence and risk factors for development of venous thromboembolism in Indian patients undergoing major orthopaedic surgery: Results of a prospective study. Postgrad Med J. 2006;82:136-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149:209-20.
- [CrossRef] [PubMed] [Google Scholar]
- RAVS Study: An Indian single center analysis of patients with VTE. Ann Vasc Dis. 2019;12:205-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715-22.
- [CrossRef] [PubMed] [Google Scholar]
- Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): A multinational cross-sectional study. Lancet. 2008;371:387-94.
- [CrossRef] [PubMed] [Google Scholar]
- Is thromboprophylaxis really justified among Indian population with femur shaft fractures treated with IM nailing? Int J Orthop Sci. 2018;4:492-5.
- [Google Scholar]
- Postoperative deep-vein thrombosis in Asian patients is not a rarity: A prospective study of 88 patients with no prophylaxis. J Bone Joint Surg Br. 1996;78:427-30.
- [PubMed] [Google Scholar]
- European guidelines on perioperative venous thromboembolism prophylaxis. Eur J Anaesthesiol. 2018;35:142-6.
- [CrossRef] [PubMed] [Google Scholar]
- Deep vein thrombosis after total hip arthroplasty in Indian patients with and without Enoxaparin. J Orth Surg (Hong Kong). 2004;12:173-7.
- [CrossRef] [PubMed] [Google Scholar]
- Deep vein thrombosis in indian cancer patients undergoing major thoracic and abdomino-pelvic surgery. Indian J Surg Oncol. 2016;7:425-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Arrive: A retrospective registry of Indian patients with venous thromboembolism. Indian J Crit Care Med. 2016;20:150-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of deep vein thrombosis in patients with lower limb trauma. J Clin Orthop Trauma. 2016;7(Suppl 2):220-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Venous Thrombo-embolism in India. Eur J Vasc Endovasc Surg. 2009;37:482-5.
- [CrossRef] [PubMed] [Google Scholar]
- Routine chemoprophylaxis for DVT in Indian patients. Indian J Orthop. 2007;41:188-91.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparative study of extended versus short term thromboprophylaxis in patients undergoing elective total hip and knee arthroplasty in Indian population. Indian J Orthop. 2013;47:161-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology and pathophysiology of vascular thrombosis in acclimatized lowlanders at high altitude: A prospective longitudinal study. The Lancet Regional Health - Southeast Asia. 2022;3:100016.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Venous thromboembolism Guidelines. 2018 & 2020.
- Prevention of Venous Thromboembolism in 2020 and Beyond. J Clin Med. 2020;9:2467.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The surgeon general’s call to action to prevent deep vein thrombosis and pulmonary embolism. Rockville (MD): Office of the Surgeon General (US); 2008.
- Management of venous thromboembolism. J Assoc Physicians India. 2007;55:49-70.
- [PubMed] [Google Scholar]
- Analysis of patients with venous thromboembolism in a multi-specialty tertiary hospital in South India. Indian J Vasc Endovasc Surg. 2020;7:29-33.
- [Google Scholar]
- Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE), a multinational cross-sectional study: Results from the Indian subset data. Indian J Med Res. 2012;136:60-7.
- [PubMed] [PubMed Central] [Google Scholar]
- Dysfunctional fibrinolysis and cerebral venous thrombosis. Blood Cells Mol Dis. 2017;65:51-5.
- [CrossRef] [PubMed] [Google Scholar]
- Risk stratification for venous thrombosis in post–partum women in a tertiary care setup in south India. Indian J Med Res. 2020;152:523-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute venous thromboembolism in Indian patients of isolated proximal femur fractures. J Clin Orthop Trauma. 2019;10:917-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Second consensus document on diagnosis and management of acute deep vein thrombosis: Group on aorta and peripheral vascular diseases and the ESC Working Group on pulmonary circulation and right ventricular function. Eur J Prev Cardiol. 2022;29:1248-63.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer–associated venous thromboembolism in ambulatory solid organ malignancy patients: Experience from a cancer research institute. Indian J Surg Oncol. 2021;12:246-50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Risk of postoperative venous thromboembolism in Indian patients sustaining pelvi–acetabular injury. Int Orthop. 2011;35:1057-63.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A prospective observational study to determine rate of thromboprophylaxis in oncology patients undergoing abdominal or pelvic surgery. Indian J Surg Oncol. 2021;12:279-85.
- [Google Scholar]
- Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch Intern Med. 1998;158:585-93.
- [CrossRef] [PubMed] [Google Scholar]
- VTE in cancer patients – magnitude of problem, approach, and management. Indian J Cancer. 2017;54:308-12.
- [CrossRef] [PubMed] [Google Scholar]
- Real world data on clinical profile, management and outcomes of venous thromboembolism from a tertiary care centre in India. Indian Heart J. 2021;73:336-41.
- [PubMed] [Google Scholar]
- Role of age, sex, and specific provoking factors on the distal versus proximal presentation of first symptomatic deep vein thrombosis: Analysis of the SWIss Venous Thrombo Embolism Registry (SWIVTER) Intern Emerg Med. 2022;17:799-803.
- [CrossRef] [PubMed] [Google Scholar]




